AN AUDIT OF DISCHARGE PRESCRIPTIONS FOR SURGICAL AND MEDICAL PATIENTS WITH A QUALITY IMPROVEMENT INITIATIVE (submitted in 2019)
Pdf

European Statement
Patient Safety and Quality Assurance
Author(s)
Eva Heffernan, Deirdre Smith , Avril Tierney, Louise McDonnell
Why was it done?
Transitions of care such as hospital discharge present an opportunity for medication error. Lapses in communication at this interface are common. For the next healthcare provider (HCP) to issue the correct medication safely and in a timely manner, the discharge prescription needs to bridge this communication gap. Prescribing errors are the most frequent subtype of medication errors and can be repeated systematically for prolonged periods. Detection of medication error using tools such as audit, learning from these errors and planning corrective action is essential to building safer healthcare systems.
This study adapted the Health Information and Quality Authority (HIQA) national standard for patient discharge summaries to create a benchmark for discharge prescriptions in SVPH. A QI initiative targeting prescribers was developed. This was designed as a bundle intervention and was called the Discharge Prescription Education Bundle (DPEB).
What was done?
The aim of this project was to evaluate the current level of discrepancies on discharge prescriptions for surgical and medical patients and to ascertain if a quality improvement (QI) initiative can impact on the severity of medication error at the point of discharge.
How was it done?
Uncontrolled consecutive baseline and re-audit of discharge prescriptions on a 26-bed mixed medical and surgical ward. The baseline audit assessed 70 patients’ discharge prescriptions. Deviations from the standard were termed discrepancies. Discrepancies were divided based on capacity to cause error (NCC-MERP Category A) and error occurred (NCC-MERP Category B-I). Discrepancies where an error occurred (NCC-MERP Category B-I) were reported using the in-house medication incident reporting (MIR) system and dually assessed by an independent panel and the project lead for potential to cause harm. The QI initiative was implemented and its impact assessed with a re-audit of 70 patients’ discharge prescriptions.
What has been achieved?
The overall number of discrepancies reduced from 156 in the baseline to 59 in the re-audit (p<0.05). Overall compliance with the audit standards improved from 17.1% to 54.3% (p <0.05). In the baseline audit 22.8% (n=16) of patients had a discrepancy where an error occurred; this reduced to 2.65% (n=2) in the re-audit (p<0.05). The severity of errors reduced in the re-audit.
What next?
The QI initiative used was proactive not reactive. Use of the discharge education bundle was not restricted to pharmacy opening hours.
This initiative was very low cost to implement. Following on from the successful results of this project one component of DPEB called the discharge prescription visual prompt is now preprinted on all SVPH discharge prescriptions as a reminder to prescribers.
IMPLEMENTATION OF A MEDICATION RECONCILIATION PROGRAMME UPON DISCHARGE (submitted in 2019)
Pdf

European Statement
Patient Safety and Quality Assurance
Author(s)
José Marco-del Río, María Luisa Ibarra-Mira, Gregorio Romero-Candel, Ana Ramirez-Córcoles, Ana Valladolid-Walsh, Francisco Tomás Pagán-Nuñez
Why was it done?
Our main goal was to improve patient’s safety, because we noticed that many patients did not take actually all the drugs that were prescribed by the physicians, and other times there were drugs that the patients were taking because they had an active prescription, but they were not supposed to. Additionally, we aimed to improve the drug-related information that the patients take home.
What was done?
A programme which includes every patient admitted into the Internal Medicine department. It consists of three steps: clarification of chronic medication that the patients are taking, we handle them and updated schedule of their drugs upon discharge and we check the coherence with the active prescriptions.
How was it done?
We interview the patients during the admission in order to clarify and update the chronic medication that they are taking. When a patient is about to be discharged, the nurses call us, so at this moment we talk to the physician to know what changes are going to be made on the medication. To coordinate with the physicians and nurses, we had two meetings in which we established the timing of the programme, so the patients don’t have to wait too long for us. When we know the changes that the physician is going to make, we update the medication schedule to handle it to the patients or their family, and we explain to them the changes and how they should manage the new drugs. If any discrepancy or medication-related problem is detected, we talk to the physician to solve it.
What has been achieved?
In the last four months, we performed 180 discharges and we solved together with the physicians 20 discrepancies. Patients are now receiving more comprehensive information about their treatment.
What next?
To continue with the programme and broaden it to the rest of our hospital departments. Also we are working on a way of uploading our pharmacy schedules to the electronic medical record of the patients, so they can be available for every healthcare worker, which would improve even more the transitions of care.
PROCEDURE TO ENSURE CORRECT MEDICATION MANAGEMENT IN THE PERIOPERATIVE PROCESS (submitted in 2019)
Pdf

European Statement
Patient Safety and Quality Assurance
Author(s)
Noelia Vicente Oliveros, María Muñoz García, Álvaro Ruigomez Saiz, Montserrat Ferre Masferrer, Teresa Bermejo Vicedo, Eva Delgado Silveira, Lucía Quesada Muñoz, Ana María Alvarez-Diaz
Why was it done?
An analysis of the indicators of the perioperative process reflected the need to improve their quality. One of the causes of scheduled surgery cancellation was the lack of the follow up of the anaesthetist’s medication recommendations. Medications need to be carefully managed to prevent perioperative complications.
What was done?
We designed and implemented a flow chart to ensure the patient compliance of anesthetist’s medication recommendations prior to surgery. We designed a protocol for the perioperative medication management.
How was it done?
A multidisciplinary group was formed with the management of the hospital and representatives of all the services involved in the perioperative process. The group designed the flow chart of the process by consensus. Patients were candidates to enter in this process if they were on treatment with anticoagulant or 2 or more medications from the following groups: antiplatelet, antihypertensives, antidiabetics. A pharmacist called by phone three times (the day before, the day of medication change, and the day after) to the patient to ensure the compliance of anaesthetist recommendations. If there was a lack of compliance, the pharmacist contacted the surgeon who was in charge of deciding if the surgery procedure continued as scheduled. Moreover, the domiciliary medication of these patients were reconcilliated and recorded in their health record. Healthcare professionals could consult it during hospital stay. The group designed a protocol for the perioperative medication management with different medical specialists.
What has been achieved?
The project started in April 2019. The pharmacist called patients with scheduled surgery of lower limbs. A total of 31 patients benefited from the new flow chart. The pharmacist detected 38 medication errors; two involved errors concerning the suspension of anticoagulant drugs prior to surgery and four implied antihypertensive drugs. Once, it was necessary to contact the surgeon. In this case, the surgeon decided to continue with the surgery as schedule. Fifty-seven medications suffered a change in the period between the anaesthestic visit and the surgery, nine of them belonged to the monitored medication group.
What next?
The next steps are to spread the flow chart to other patients, to distribute the protocol among hospital healthcare professionals and to implement a procedure for the reintroduction of the modified medication.
DRUG SERIALISATION: ORGANIZATIONAL AND ECONOMICAL IMPACTS FOR HOSPITAL PHARMACIES (submitted in 2019)
Pdf

European Statement
Selection, Procurement and Distribution
Author(s)
Quentin HIVER, Agathe ROGER, Marine EGOT, Ivan VELLA, Marie-Hélène TYWONIUK
Why was it done?
Community and hospital pharmacists are required to apply the European directive on falsified medicines. In France, we are currently undergoing a transition phase for the progressive generalisation of serialisation. French pharmacies are more or less ahead of schedule for the implementation of decommissioning. In our pharmacy, the decommissioning has been operational since February 2019. After 8 months of practice, we are able to provide data as a basis for work and thinking.
What was done?
Determining and evaluating, by feedback approach, the organisational and economical impacts of drug serialisation for a hospital pharmacy
How was it done?
• Step-by-step description of the supply chain after implementation of decommissioning. • Collection of the man-hours necessary for: decommissioning implementation, software training, routine decommissioning, problem solving. • Census of financial investments
What has been achieved?
After analysis of our supply chain, the reception stage appeared to be the most favorable for decommissioning, in terms of practicality, safety and traceability. Several steps have thus been added at reception: Identification of serialized boxes, manual scan, checking of the decommissioning report and the number of decommissioned boxes, printing of the report. The pharmaceutical time necessary for the decommissioning implementation has been estimated to up to 28 hours. The software training was made in small groups of 2−3 agents, requiring 9 minutes per agent on average. The decommissioning is currently requiring 17 minutes for 100 boxes. Over 8 months, the time necessary for the pharmacists to solve problems linked with serialisation (non-operational Hub, corrupted database, error message at decommissioning…) was estimated to up to 7 hours. The financial investment amounts to 17200 euros (software+ergonomic desk+man-hours at implementation).
What next?
The decommissioning itself doesn’t have a major impact on the pharmacy’s organization. But, ensuring a clear and safe supply chain, to identify which boxes must be decommissioned and which boxes can be dispensed, is time-consuming. It goes through a proper working environment with a forward supply chain and traceability tools. Moreover, the encountered problems were mainly due to computer failures, requiring a performing software with an efficient maintenance. We are currently working on improving the ergonomics of the workstation to avoid the risk of musculoskeletal disorders due to decommissioning.
IMPLANTATION OF A COMMUNICATION CIRCUIT OF ALERTS AND SAFETY NOTES RELATED TO DRUGS FROM THE PHARMACY DEPARTMENT (submitted in 2019)
Pdf

European Statement
Patient Safety and Quality Assurance
Author(s)
Ignacio García Giménez, Natalia Martín Fernández, Olalla Montero Pérez, Ernesto Sánchez Gómez, Isabel María Carrión Madroñal
Why was it done?
The aim is to implement a protocol to follow when these safety notes/alerts are released from the AEMPS. It comprehends the reception of the information, its registration and its communication, when needed, to the rest of the healthcare professionals.
What was done?
A communication circuit of alerts and safety notes related to drugs coming from the “Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)”.
How was it done?
At the reception of an alert from the AEMPS, the first step is to check if the drug has been acquired by the Pharmacy, and then act in accordance with the recommendations, informing the Departments in which the medication had been dispensed. If a drug must be retired and a stock break is generated, the healthcare professionals must be informed as well. Security notes from the AEMPS are published in the local hospital website, where the documents sent by the AEMPS can be found. If this medication is included in the Pharmacotherapeutic guide, a notification is shown when it is prescribed. Finally, all alerts and security notes, with the pharmacist intervention, are registered in a database.
What has been achieved?
Since the implementation of the circuit, 14 alerts and 9 security notes were sent from the AEMPS in a period of 6 months. No interventions regarding the alerts were needed. Healthcare professionals were informed when the security notes were released, pointing to the patients at risk, the precautions required and the alternative therapies available.
What next?
To incorporate it as an indicator of quality of care within the procedures performed by the pharmacy department and detect areas of improvement.
SAFE PRESCRIBING METRICS FOR HOSPITAL PHARMACY (submitted in 2019)
European Statement
Patient Safety and Quality Assurance
Author(s)
Oran Quinn, Anna Marzec
Why was it done?
Errors of miscalculation, doses inappropriate for renal function and at extremes of weight were reported when doses of medication were written as ‘mg/kg’ without stating the dose to be given e.g. Gentamicin 5mg/kg, Vancomycin 15mg/kg and Enoxaparin 1.5mg/kg.
What was done?
A quality improvement initiative to resolve issues with prescribing medications dosed by weight. Nursing staff were identified as ‘gate-keepers’ who could refuse to administer medication inappropriately prescribed. Identification, agreement, education and feedback were necessary to change prescribing practice and support nursing staff. Hospital doctors were required to calculate and prescribe the total dose to be given. Feedback was given by monthly bulletin.
How was it done?
Support from key stakeholders was sought to endorse the initiative. Verbal and written education was given to nursing, medical and pharmacy staff to implement the initiative on an agreed date. Refusal to administer medication unsafely prescribed was key to successful implementation. Patient’s weight was not always available and additional equipment was provided to overcome this problem. The risk of withholding treatment was considered and an escalating referral process was recommended contacting the Senior House Officer, then Registrar and ultimately the patients Consultant to avoid lengthy delays to patient treatment. Nurses felt supported in refusing to administer medication.
What has been achieved?
A point prevalence study of all inpatients was carried out monthly to ascertain the level of compliance Mar-19 Apr-19 May-19 Jun-19 Jul-19 Aug-19 % of patients with total dose prescribed correctly 67.0 86.7 96.7 100.0 100.0 88.9 87.5. Results showed overall improvement from March to August and full compliance in May and June. Success was achieved through a multidisciplinary approach involving all key stakeholders, a forcing function and support from and for front line staff.
What next?
This initiative has been further developed to become ‘Monthly Safe Prescribing Metrics’.
Other prescribing metrics such as using ‘iu’ dosing for Insulin, prescribing appropriately for patients at extremes of weight and using the abbreviation ‘mcg’ for medications dosed in ‘micrograms’ were included. Initiatives to improve all metrics are ongoing.
Safe prescribing metrics could help to positively influence prescribing culture in other healthcare settings.
HERA – A NEW TOOL FOR THE QUALITATIVE AND PHARMACOECONOMICAL EVALUATION OF GENERIC DRUG PRODUCTS BEFORE CHANGING BRANDS (submitted in 2019)
Pdf

European Statement
Selection, Procurement and Distribution
Author(s)
Steffen Amann, Rudolf Bernard, Georg Berndt , Meike Bindemann, Myga Brakebusch, Jörg Brüggmann, Frank Dörje, Miriam Gyalrong-Steur, Anita Kellermann, Markus Müller, Elfriede Nusser-Rothermundt, Rainer Riedel, Eva Tydecks
Why was it done?
Given rising cost-pressure and increasing numbers of supply shortages, changes between generics have become daily practice in hospital pharmacies. To ensure constant treatment quality and patient safety, the equivalence of a potential new product with the current one must be guaranteed before changing brands. So far there has been no transparent, standardised tool for the comparison of generics workable in everyday clinical practice. Developing such a tool was our project’s aim.
What was done?
We developed an Excel-based tool for the qualitative and pharmacoeconomical evaluation of generics before changing brands (aut-idem substitution) in hospitals.
How was it done?
A working-group of pharmacists from seven hospitals developed the “HERA” tool (HTA-evaluation of geneReric phArmaceuticals). Starting from a base version, 22 generic products were assessed with the tool during five evaluation rounds. Based on these results the instrument was gradually refined. Within HERA‘s Excel matrix a potentially to-be-used generic is compared with the current one. The economic evaluation is based on unit prices and prescription volumes, but also includes process costs associated with the product change. The assessment of pharmaceutical quality is based on 34 criteria from six areas (licensed uses, drug substance, dosage form and excipients, handling, safe design, packaging and storage). The objective quality evaluation is complemented by the assessment of hospital-specific features. Complex substitutions – e.g. associated with a handling change – require involvement of the medical staff using the product. The purchasing decision is taken based on the synopsis of pharmaceutical quality and economic evaluation.
What has been achieved?
The standardised evaluation of product differences before substitutions allows for the early identification of potential problems of brand changes and helps avoiding them for the benefit of patient safety. HERA also guarantees reproducibility and transparent, QM-compliant documentation of product changes. The pharmacies of our purchasing group now routinely use HERA for the assessment of generics before intended brand substitutions. Each evaluation is conducted in one pharmacy and shared with the others via data-cloud.
What next?
We have published a paper on HERA and presented it at the German Hospital Pharmacists congress in 2018. Our aim is to create a network of colleagues with shared access to all colleagues’ HERA product evaluations to reduce the workload for the individual pharmacies.
THE IMPACT OF A WARD SATELLITE PHARMACY ON CLINICAL PHARMACY SERVICES AND POTENTIAL COST BENEFIET (submitted in 2019)
Pdf

European Statement
Patient Safety and Quality Assurance
Author(s)
Thewodros Leka, iun Grayston, Mashal Kamran, Biljana Markovic
Why was it done?
The Carter report recommended that about 80% of hospital pharmacist time should be spent on the wards to provide clinical pharmacy services. However, in our hospital’s surgical specialty at the time of this report, it was found that only 33% of pharmacist’s time was spent on clinical pharmacy services. This had a negative impact on:
• rate of medication errors and near misses
• supply of critical medicines
• pharmacist participation in productive ward rounds
• timely discharge of patients home
What was done?
The Pharmacy department made a successful business case to the Hospital executives to open a Satellite pharmacy to serve 4 surgical wards. The proposal was to recruit a dedicated clinical pharmacist and Medicines Management Technician, and set-up a dispensing satellite pharmacy.
How was it done?
The business case indicated that if funded, the new satellite pharmacy team would: • improve clinical pharmacy key performance indicators • improve patient safety • deliver a potential cost benefit Funding limitation was an obstacle and we have to convince the board.
What has been achieved?
We achieved 60−90% improvement in the objectives set in the business case as illustrated in Table 1 and 2. The pharmacy team won the annual quality improvement award of 2018. Table 1: Clinical Pharmacy Service improvement Clinical pharmacy services Service rate pre-satellite pharmacy Service rate post satellite pharmacy % of service improvement Medication errors 16/month 6/month 63% Pharmacist interventions 20/month 80/month 75% Pharmacist participation in ward round 6/month 50/month 88% Time to dispense discharge summaries 90 minutes/discharge summary 20 minutes/discharge summary 77% Number of patients counselled 15/month 75/month 80% Pharmacist available in the ward 1.5 hrs/day 7.5 hrs/day 80% Time taken to supply critical medicines 1 hour 5 minutes 91% Table 2: Potential Cost-benefit savings achieved Activities Cost-benefit savings/year (€) Reducing length of stay of patients €17,000 Reducing repeat dispensing €16,000 Effective use of nursing time €11,000 Reducing prescribing errors €103,000 Total Savings €147,000.
What next?
• Weekend working.
• Service improvements can be transferred to acute medical units and downstream medical wards. Reference Carter report.
THE RISK MANAGEMENT OF THE PHARMACY PREPARATIONS IN THE HOSPITAL PHARMACIES (submitted in 2019)
Pdf

European Statement
Production and Compounding
Author(s)
ADRIANA DURCANSKA
Why was it done?
The quality and safety standards of pharmacy preparations are not harmonised throughout Europe. They fall under the national competencies of individual European countries.
What was done?
The quantitative risk assessment of the pharmacy preparations for stock in hospital pharmacies (HPs) in accordance with Resolution EDQM CM / Res (2016) 1; to specify the decision criteria for the risk assessment; the risk management of the pharmacy preparations for stock in the country; to design a check list of the risk assessment for extempore preparations.
How was it done?
Out of the total number of 53 hospital pharmacies contacted, 5 pharmacies sent a suitable file.
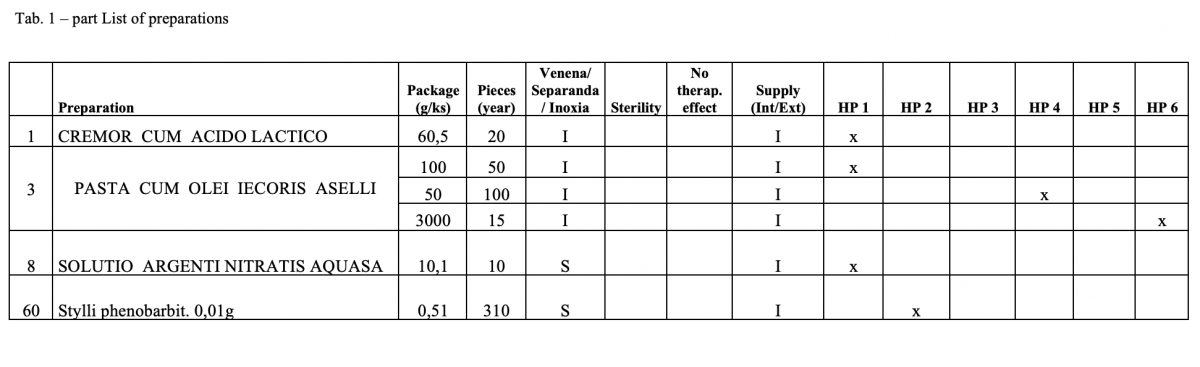
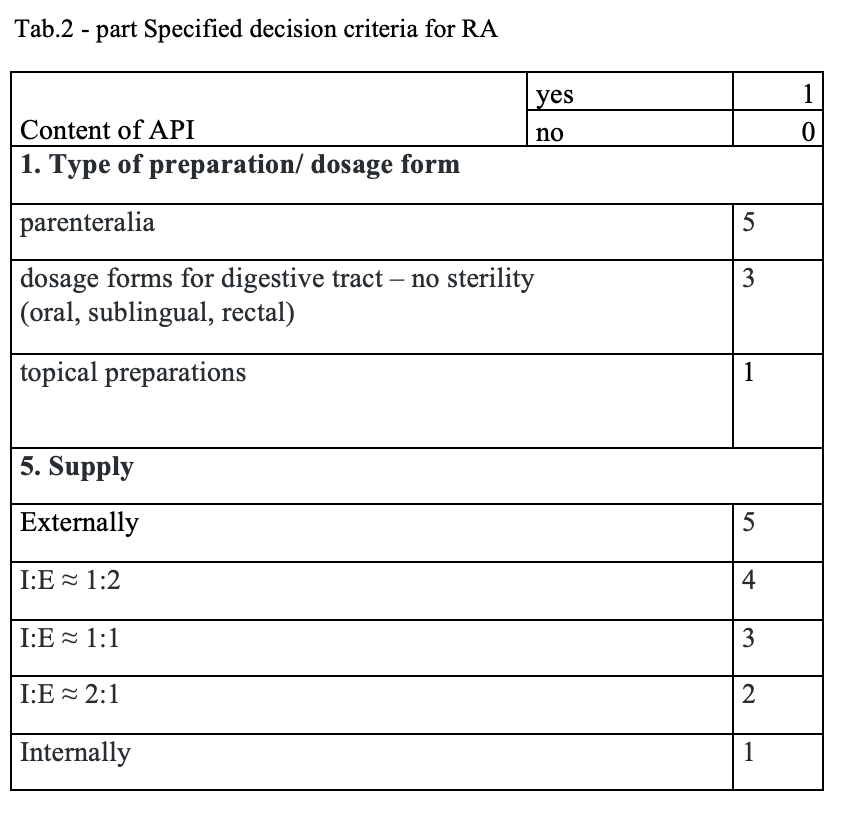
What has been achieved?
A total of 170 types of medicines are being prepared in HPs. One HP had the result of the risk ≥ 100 when preparing ophthalmic medicines. Annex A is a check list designed to assess the risk of extempore preparations.
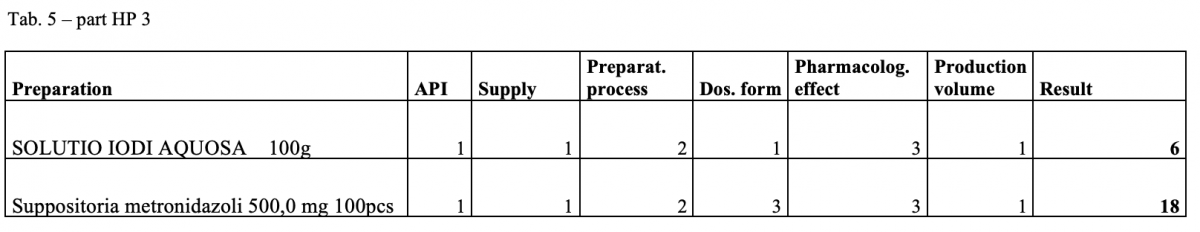
What next?
The management is and will be forced to consider its introduction or to use another model: hospital – GMP / outsourcing / central pharmacy preparing and distributing. The aim of using the document in hospital pharmacies of the country.
IMPLEMENTATION OF A MEDICATION SAFETY AGENDA AT TWO HOSPITAL SITES IN RESPONSE TO WORLD HEALTH ORGANISATION (WHO) PATIENT SAFETY CHALLENGE ‘MEDICATION WITHOUT HARM’ (submitted in 2019)
Pdf

European Statement
Patient Safety and Quality Assurance
Author(s)
Meenal Patel, Sheena Patel, Peta Longstaff
Why was it done?
• Initiative introduced and on-going since 2017
• To increase and embed medication safety awareness
• To address under-reporting of medication-related incidents, with feedback
• To embed medication safety in education programmes and clinical practice
What was done?
A local medication safety agenda implemented across two hospital sites in response to World Health Organisation (WHO) patient safety challenge ‘Medication without Harm’.
How was it done?
• Medication safety group (MSG) introduced with local strategy, involving junior medical staff for frontline feedback • Medication safety metrics changed to allow benchmarking with peers as per NHS Improvement’s Model Hospital data • ‘Plan, Do, Study, Act’ model applied to improve transfer of care from hospital to rehabilitation unit following external incidents • Monthly analysis of incidents with harm, exploring reasons for under-reporting • Optimisation of incident reporting system to improve staff feedback following investigations • Near miss error log introduced in pharmacy with shared learning • Mitigation of medication-related risks e.g. medications safe storage action plan • Medication safety bulletins, patient safety newsletters and top tips guide introduced covering focal themes • ‘Safe prescribing’ mandatory induction training for junior doctors to support prescribing of high risk medicines and compliance to patient safety alerts • Hospital-wide education on lessons learnt from incidents • Medication safety resources for staff to access • Nursing quality round on medication safety • Electronic missed doses realtime report developed to tackle omitted/delayed critical medication doses • Medication safety awareness (MSA) week held to increase awareness on focal themes
What has been achieved?
• Multidisciplinary MSG with assurance on meeting WHO global challenge. • Monthly analysis of medication safety data to allow learning, collaboration and benchmarking against peers. • Positive staff feedback on bulletins/newsletters with staff involvement/engagement. • Training programmes embedded with safe prescribing education. • Improved hospital safety metrics: Following MSA week, a 5% and 21% increase in medication-related incident reporting occurred at each site which has been sustained. Reporting rates doubled at one site following success of MSA week. • In 2018-19, local target achieved for reported medication-related incidents per 100,000 finished consultant episodes and medication-related incidents with harm
What next?
• Collaborative multidisciplinary working raising the profile of pharmacists acting as medication safety officers
• Implementing medication safety measures from NHS Patient Safety Strategy 2019
• Initiatives for safer culture, safer systems and safer patients



























