Active Pharmacovigilance of COVID-19 vaccines
European Statement
Patient Safety and Quality Assurance
Author(s)
Manuela de Sousa, Ana Catarina Felismino, Ana Rita Pereira, Ivone Máximo, Liliana Pedro, Natacha Santos, Paula Campos
Why was it done?
Vaccines against COVID-19 are classified as “Medicines subject to additional monitoring” by the European Medicines Agency, making it essential to implement active pharmacovigilance systems that allow for the rapid identification of new safety information.
What was done?
Active surveillance of the COVID-19 vaccination process of health-care professionals and immunoallergy patients of our hospital.
How was it done?
By proposal of the Pharmacy Service, a multidisciplinary Pharmacovigilance Committee composed of two Pharmacists, two Physicians and two Nurses was created. The vaccinated professionals and patients were periodically identified, with the support of the Occupational Health and Immunoallergy Departments. Questionnaires to identify Adverse Drug Reactions (ADR) were created in Google Forms® for each COVID-19 vaccine brand and sent to health-care professionals´ institutional email address, or to the email patients provided in the signed informed consent. The responses were exported to an EXCEL® database, analyzed by the Pharmacovigilance Committee, the ADRs identified and communicated to the regional Pharmacovigilance Centre.
What has been achieved?
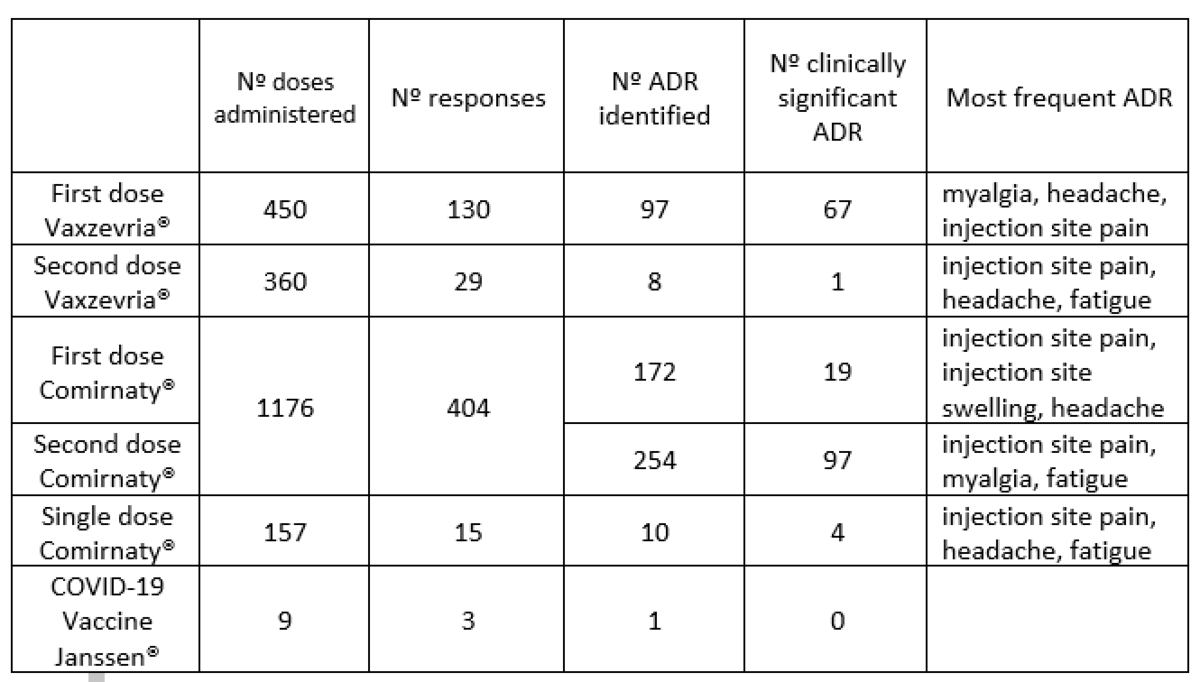
From 29 December 2020 to 31 August 2021 a total of 2141 questionnaires were sent, 578 responses were obtained and 542 ADRs were communicated to the regional Pharmacovigilance Centre. No anaphylactic reactions were reported. Nº doses administered Nº responses Nº ADR identified Nº clinically significant ADR Most frequent ADR First dose Vaxzevria® 450 130 97 67 myalgia, headache, injection site pain Second dose Vaxzevria® 360 29 8 1 injection site pain, headache, fatigue First dose Comirnaty® 1176 404 172 19 injection site pain, injection site swelling, headache Second dose Comirnaty® 1176 404 254 97 injection site pain, myalgia, fatigue Single dose Comirnaty® 157 15 10 4 injection site pain, headache, fatigue COVID-19 Vaccine Janssen® 9 3 1 0
What next?
Increase awareness of the importance of Pharmacovigilance amongst hospital health-care professionals to combat sub-notification of ADRs. We also plan to extend this active Pharmacovigilance program to other medicines in use at our hospital.

























