EXPERIENCE OF PATISIRAN OPTIMIZATION BUILDING HEALTHCARE TEAM
European Statement
Clinical Pharmacy Services
Author(s)
CRISTINA GONZALEZ PEREZ, LUCIA GALÁN DAVILA, MARTA ORTIZ PICA, ELENA GARCIA SUAREZ, MARIA MOLINERO MUÑOZ, LIDIA YBAÑEZ GARCIA, NATALIA SANCHE-OCAÑA MARTIN, MARIA DE LA TORRE ORTIZ, JAVIER CORAZON VILLANUEVA, JOSE MANUEL MARTINEZ SESMERO
Why was it done?
Our hospital is a reference in our community for this rare disease. Since patisiran was approved, we have treated 19 patients. Due to the high cost of patisiran and the few patients treated, it is necessary to optimize patient treatments in the most efficient way.
What was done?
Transthyretin (TTR) amyloidosis is a rare disease caused by mutations in the TTR gene. These mutations alter the normal function of TTR protein, creating slowly progressive condition characterized by the buildup of abnormal deposits of amyloid in body’s organs and tissues.
Patisiran is a TTR specific small interfering RNA (siRNA) formulation in lipid nanoparticles, which has been shown to substantially reduce the production of abnormal TTR in patients with hereditary amyloidosis TTR.
With this project we look for achieve the greatest possible savings from patisiran drug without affecting the patient’s efficacy.
How was it done?
We created a working group with neurology department, to define patient’s groups according to:
– Prescribed doses (doses per patient weight; 0.3 mg/kg).
– Preparation’s losses because of the filters
– Losses of the extraction process.
– Other individual conditions as posology or personal preferences.
Patients belonging to the same group have to administrate patisiran the same days.
As the patient’s weight can change along the time, we agree with doctors and nurses to weight the patients every six months to perform doses adjustments. Therefore, every six months we should regroup the patients to optimize the most.
What has been achieved?
These clusters have made possible to reduce wasting vials and therefore to permit significant savings. We estimate around 208.115 € savings/year.
What next?
With this practice we look for a more efficient and sustainable rational health system. The savings achieved can be used to treat other patients or promote new investigations. Also, it makes possible a better healthcare team performance, working together for a better attention, health quality, security, and treatments efficiency. Monitoring weights to adjust doses and closer follow-up of patients by the different members of the multidisciplinary team are examples of the achievements.
Initial observations on the implementation of a clinical pharmacy service in a rural hospital in Austria
European Statement
Clinical Pharmacy Services
Author(s)
Sonja Guntschnig, Aaron Courtenay, Ahmed Abuelhana, Michael Scott
Why was it done?
The service was established as part of the implementation of a new pharmacy into the hospital. The aim of this good practice initiative was to introduce multidisciplinary work on the wards and provide clinical pharmacy support for the ward personnel. Furthermore, it determined what types of clinical pharmacy interventions are needed at a rural 360-bed hospital in Austria, and assessed the physicians’ acceptance rate of the pharmacists’ suggestions.
What was done?
A new clinical pharmacy service (CPS) was introduced into Tauernklinikum Zell am See.
How was it done?
Data on 550 interventions made by one clinical pharmacist were collected by convenience sampling over a one-year period and rated on a six-point clinical significance scale. A subset of 26 interventions was rated for clinical significance by four independent physicians to determine inter-rater reliability (IRR). A two-way model inter-rater reliability analysis was performed for the four different physician assessments using SPSS to determine intra-class correlation (ICC).
What has been achieved?
Prompt acceptance rate by the physicians involved was 71.3% (392/550). In 26.9% (148/550) of all cases, the physician considered a change. The overall average score for all 550 clinical pharmaceutical interventions taken was 2.2. ICC significance scores were correlated with the pharmacist’s scores, ICC for consistency was 0.732 and 0.732 for absolute agreement, thus both can be considered as “good”. Potential for cost reduction associated with the recommended pharmaceutical changes, namely with medication being stopped or dose reduction was 32.7% (180/550) and 25.1% (138/550), respectively.
What next?
There is great potential and a definite need for the expansion of CPS in Austria. Only 15.8% of Austrian hospitals have a pharmacy department with even less offering CPS. Many countries have demonstrated the benefits of CPS in hospitals over the past 30 and more years. The need for increased pharmacist staffing in Austrian hospitals needs to be demonstrated to Austrian stakeholders.
ORAL IVERMECTIN EFFECTIVENESS IN THE TREATMENT OF PERMETRIN-RESISTANT SCABIOSIS: A DESCRIPTIVE, RETROSPECTIVE AND OBSERVATIONAL STUDY
European Statement
Clinical Pharmacy Services
Author(s)
Emilio Monte-Boquet, Mª Jesus Cuellar-Monreal, Mª Vicenta Tarazona-Casany, Ana Alejandra Garcia-Robles, Eduardo Guerrero-Hurtado, Inmaculada Beltran-Garcia, Patricia Polo-Montanero, Antonio Solana-Altabella, Jose Luis Poveda-Andres
Why was it done?
Ivermectin is used as a therapeutic alternative for permethrin-resistant scabies. The recommended treatment consists of administering two single doses (SD) separated by 7-14 days. An increased incidence and resistance to permethrin was observed in late 2020 possibly influenced by SARS-CoV-2 pandemic.
What was done?
To assess the effectiveness of oral ivermectin as a treatment for topical 5% permethrin-resistant scabies in patients from a tertiary hospital and to analyze the characteristics of the sample and the treatment.
How was it done?
An observational, retrospective and descriptive study was done including patients who collected Ivermectin 3mg tablets in the Outpatient Pharmaceutical Care Unit of the hospital between April 2020 and April 2021. All patients were previously treated with 5% permethrin and had failed. Treatment with ivermectin was considered effective in patients who were discharged from Dermatology Clinics or did not consult for itching or other symptoms 4 weeks after the last dose. Other variables were: number of doses received, age, sex and antecedents within family nucleus or cohabiting cases were also collected.
What has been achieved?
A total of 37 patients were included and 39 applications were made. There were 16 applications from April 2020 to December 2020 (mean of 1.78±1.79 applications/month [95%CI:0,41-3,05), and 23 from January to April 2021 (mean of 4,6±2.6 applications/month [95%CI:1,37-7,83]). Ivermectin was effective in 87,2%(34/39) patients and in the remaining 12,8%(5/39) therapeutic failure happened, so they required treatment for a second time. A patient was excluded because it was unclear if treatment had been ineffective or reinfestation happend. The 56,4%(22/39) of patients received two SD separated for 7–14 days. The 58,5%(24/39) of patients were women and the mean age of the sample was 31,1±19,3 years old (95%CI:26,8-37,4). The 54,0%(21/39) of the patients had between 11-30 years old, and the 74,4%(29/39) had a history or cohabitants within their family nucleus.
What next?
In our study sample, effectiveness of Ivermectin was greater than 90% in scabies resistant to topical 5% permethrin and seems independent of the number of doses received. Results suggest that scabies mainly affects women and Young people. Infections in cohabitants seem to have an increased frequence and may had been influenced by confinament and delays of treatments during SARS-CoV-2 pandemic.
ANTIMICROBIAL DOSING IN OBESE PATIENTS: ADJUSTMENT GUIDE TO HELP IN PRESCRIPTION AND VALIDATION
European Statement
Clinical Pharmacy Services
Author(s)
ANA CASTRO BALADO, MARÍA TERESA RODRÍGUEZ JATO, IRIA VARELA REY, MANUEL BUSTO IGLESIAS, FRANCISCO CAJADE PASCUAL, MARIA PUENTE IGLESIAS, IRENE ZARRA FERRO
Why was it done?
The prevalence of obesity has increased considerably in last decades. Pathophysiological changes in obese patients (body mass index >30kg/m2) produce pharmacokinetic and pharmacodynamic alterations that can condition the correct exposure to drugs if standard dosages are used. Incorrect dosing in obese patients can lead to toxicity or therapeutic failure. Usually, it is difficult to find information on drug dosing in this population, being sometimes contradictory.
What was done?
To develop an antimicrobial dosage infographic guide for obese patients to facilitate the correct prescription and validation in our hospital.
How was it done?
Initially, the pharmacy service established the antimicrobial groups to be included in the infographic guide, and within each of them, the most used in hospitalized patients were identified. A bibliographic search was carried out using the keywords “obese”, “dosing”, “antimicrobial” and “patient”. Technical data sheets, PubMed, Google Scholar, UpToDate, Micromedex and other hospital guidelines were consulted. A peer review was carried out and a table with antimicrobial groups and subgroups was designed, stablishing the body weight recommended for the adjustment if needed (total weight (TBW), ideal weight (IBW) and adjusted weight (ABW)), doses, and observations (monitoring of plasma levels, administration in extended/continuous infusion and/or special considerations). The table consisted of a total of 38 antibiotics, 10 antifungals, 6 antivirals and 4 antituberculous drugs. Of the total antimicrobials reviewed, 40% were dosed in obese patients by maximum dose, 38% were dosed based on weight (21% by ABW, 10% by IBW, and 7% by TBW) and 22% by standard doses. Equations for body weight descriptors were added in the infographic.
What has been achieved?
The protocol was validated by the hospital’s infectious diseases commission. Dosing information was then entered into the hospital’s electronic prescribing program facilitating the correct antimicrobial prescription and validation in obese patients. Training was provided in the intensive care unit, and the final document was disseminated through the hospital intranet and via email to all prescribing physicians in the center, being well embraced.
What next?
We will continue making formative sessions, as well as possible updates that may be beneficial for a better antimicrobial dosing in obese patients to avoid toxicities and/or therapeutic failure.
An interprofessional team for the management of nausea and vomiting in a haematological oncology unit
European Statement
Clinical Pharmacy Services
Author(s)
Mapi Fleury, Januska De Maria-Lee, Alessandra Taiana, Yvan Bourgeois, Sophie Voruz, Olivier Spertini, Pierre-Yves Bochud
Why was it done?
In 2019, procedural changes within unit treating malignant haemopathies raised awareness about unsatisfactory management of NV, particularly CINV. We identified a lack of departmental consensus, leading to heterogeneous therapeutic practices, confusion over the aetiologies of NV and feelings of powerlessness among healthcare professionals. We decided to improve the whole process, from prophylaxis to treatment, by addressing specific knowledge gaps concerning CINV, improving pathophysiological and pharmacological knowledge, and implementing interprofessional management and MASCC/ESMO guidelines.
What was done?
Patient nausea and vomiting (NV)—particularly chemotherapy-induced NV (CINV) in malignant haemopathies—was managed by an interprofessional team.
How was it done?
A multidisciplinary working group was established to create a comprehensive, patient-centred NV management programme. Work sessions focussed on attaining a therapeutic consensus and adapting international guidelines to our context. Different professions learnt about each other’s needs and fields of competency, enabling each to be heard and creating mutual benefits through sharing expertise and knowledge. Pathophysiological/pharmacological leadership was given to clinical pharmacist, including developing and teaching specific protocols and supervising complex clinical situations in the field.
What has been achieved?
Interprofessional consensus was reached, documentation and techniques were implemented including clinical evaluation checklists at patient admission. CINV therapeutic regimens were completely updated and immediately and automatically included in cancer treatment protocols. The clinical pharmacist and specialist nurses give initial interprofessional training to new colleagues and ensure continuous on-site supervision. This transversal work has resulted in fewer patients suffering from NV and better team understanding of pathophysiological mechanisms, differential diagnoses and adverse drug effects—this also ended the use of unsuitable medications and dosages. Overcoming this critical situation also allowed us to begin non-pharmacological integrative care.
What next?
Interprofessional working group proved indispensable to this approach. Including CINV pharmacotherapy directly into cancer treatment plans is one of programme’s strong points and contributes to high adherence to guidelines. Team feels more relaxed and more in control. Monitoring is now done by tracking files and oral feedback, but we aim to implement systematic follow-up of interventions, care evaluations and NV to assess programme’s impact. Next stage will also include patient feedback.
New frontiers of hospital pharmacy: management and preparation of human tissues used in the surgery room
European Statement
Clinical Pharmacy Services
Author(s)
Andrea Ossato, Giuseppe Giovagnoni, Michele Giannini, Anna Francesca Spada, Francesca Realdon, Valeria Mezzadrelli, Lorenza Cipriano, Nicola Realdon, Teresa Zuppini, Roberto Tessari
Why was it done?
Since 1st October 2019, the regional tissue bank that supplies hospital, stopped sending ready-made tissue to the implant, preferring the shipment of tissues frozen at -80°C. For this reason, the hospital pharmacy developed a procedure for the management of orthopedic allografts ensuring a clear and safe supply chain reducing the waste raised from the obligation of immediate use of the thawed tissue.
What was done?
Hospital pharmacists, in agreement with the hospital administrators and the orthopedic surgery department, developed a new service characterized by procurement, processing, preservation, storage, thawing and preparation of human tissues and cells for orthopedic allografts, according to European and national legislation.
How was it done?
The management of orthopedic allografts took place as follows: was established a dedicated path for communications with orthopedic surgery and bank tissue; tissue thawing and washing was centralized in the clean-room of the hospital pharmacy and were guarantee adequate training of all personnel involved as well as complete standard operating procedure documentation for all stages of the process and appropriate control measures.
What has been achieved?
Evaluation of the process showed that it was favourable in terms of practicality, safety, traceability and cost saving. Especially, the centralization of tissue preparation within clean‐rooms with aseptic technique, allows microbiologically safer setups reducing clinical risk. A further guarantee of safety is given by the sterility process’s validation through Media Fill test. This organisation allowed us to reduce the waste through a more effectively management of the tissues shelf life and any missed surgery with a cost saving and an ethical behaviour.
What next?
Optimise patient outcomes through working collaboratively within multidisciplinary teams and using the limited health systems resources responsibly, are two main goals expressed by the last European Statements of Hospital Pharmacy (ESHP). This study demonstrated how the centralization of tissues management in the hospital pharmacy make the process more efficient and safer and thus comply with the ESHP’s goals; leading to a clinical advantage for patients and better economic impact for the hospital.
Pharmaceutical care to Pediatric Home Health Care
European Statement
Clinical Pharmacy Services
Why was it done?
Home Health Care is a new emerging model of health care, with a great impact on pediatrics.
Pharmaceutical care is relevant in these population because:
•The pediatric patient,for many reasons, involves difficulties in the use of medications (adaptation of pharmaceutical forms, preparation of magistral formula, off-label use, need for calculations, etc.).
•Health education is esencial to family/primary caregiver of patients admitted at home
What was done?
Reciently a pharmacist has joined to multidisciplinary Home Health Care team.
How was it done?
Pharmaceutical Care consists on:
• Clinical and pharmacotherapeutic daily follow up
• Medication reconciliation for polymedicated patients, with narrow therapeutic range drugs, or chronic diseases (oncological, neurological…)
• Pharmaceutical validation, verifying: the indication, dosage, route of administration, drug interactions, adequacy of the dosage form to the patient’s situation
• Compounding sterile preparations at Pharmacy Service. It allows a longer storage period of them, so it will reduce nursing/medical visits to home in patients with stable health condition, so the unit can admitted more patients
• Active participation in multidisciplinary sessions to advise on pharmacological issues and ensure the maximum efficiency and safety of the treatments
• Dispensing weekly of prescribed medication
• Registration of pharmaceutical interventions and cost saving by compounding sterile preparations
What has been achieved?
The average of pharmaceutical interventions during six months were 17,5 per month, 90.7% were accepted. It means that 57,4% of admited patients to Pediatric Home Health Care Unit were done a pharmaceutical intervention.
The types of pharmaceutical interventions were: 35,3% for dosing of drugs, 27% for pharmacokinetic monitoring, 18% for medication use, 4% by prescription error, 4% for preparation and administration of drugs at home. Others were about monitoring side effects and medication acquisition.
Finally, 657 sterile preparations were compounding at Pharmacy Service, it has involved a cost saving of 5143€.
What next?
It is neccesary an individualized pharmaceutical care to chronic, polymedicated and pluripathological pediatric patient in Home Health Care Unit. It will be performed:
• Clinical and pharmacotherapeutic telematic follow up
• Telephone/telematic assistance with the pharmacist for any doubts about the use of drugs
• A personalized report with individualized recomendations about preparation, administration, manipulation, elimination and acquisition of drugs.
The design and implementation of a crushability algorithm: first experiences of a pharmacist-lead medication review in patients with swallowing difficulties
European Statement
Clinical Pharmacy Services
Author(s)
Nicolas Sagaria, Daniele Mengato
Why was it done?
Dysphagia is a swallowing disorder more common in the elderly. The pharmaceutical market is not always able to meet the needs of dysphagic patients and manipulation of a medication is sometimes the only possible way to ensure its administration. This activity is often delegated to nurses or other professionals without the necessary education.
What was done?
The manipulation of solid oral pharmaceutical forms, such as tablets or capsules, is such a common act that it is sometimes regarded superficially. In order to guide the clinical pharmacist in assessing the crushability of a tablet, we designed, and validated through an on-field application, a decision-making algorithm.
How was it done?
The first two steps of the algorithm help, respectively, to understand whether there are clear indications in the summary of product characteristics on how to manipulate the drug and/or whether there are alternatives on the market, other than the solid oral, suitable for administration to the dysphagic patient. When neither of these steps is feasible, the pharmacist is guided towards direct manipulation of the drug, supported by evidence in literature and study of excipients. If a solution cannot be found, the physician should be advised to consider a switch or a discontinuation of therapy. Finally, we tested the algorithm by including it in a medication review form in a otolaryngology-ENT department, where the incidence of dysphagic patients was higher.
What has been achieved?
In the second half of 2020, we analyzed 45 medrev forms filled out in the ENT-department. Each form contained an average of 2.8 drugs to be re-analyzed for the dysphagic patient. We applied our algorithm to a total of 123 drugs. For 101 (82%) of these, we provided precise information on the correct way to administer and manipulate the drug. For only 22 drugs, a discussion with the prescriber was necessary to identify an alternative. In this way we have improved patients’ and operators’ safety.
What next?
In the near future we will expand our test to other departments, not only surgical, and try to minimise the rate of drugs for which we could not provide information on manipulation. In addition, we are planning to develop a software to simplify the process.
OPTIMIZATION AND CENTRALIZATION OF THE HANDLING CIRCUIT OF HAZARD DRUGS FROM THE PHARMACY SERVICE
European Statement
Clinical Pharmacy Services
Author(s)
Mireia Iglesias Rodrigo, Júlia Pardo Pastor, Alba Manzaneque Gordon, Cristina Sangrador Pelluz, Núria Meca Casasnovas, Clara Sebastián Carrasco, Fernando Salazar Gonzalez, Gemma Garreta Fontelles, Jordi Nicolás Picó
Why was it done?
Due to the risk posed by the handling of Hazard Drugs (HD) in the healthcare field, it is necessary to implement circuits that guarantee the professional’s safety.
What was done?
Create an internal classification of HD based on the NIOSH List of Hazard Drugs in Healthcare Setting 2020, to optimize the circuit of its handling from its receiving to its administration.
How was it done?
Considering the HD included in the Pharmacotherapeutic Guide (PTG) of our center, the stages of reception and adaptation/preparation/dispensing/administration and other precautions were analyzed.
Categories were established, analyzing the needs of each stage according to: NIOSH level of danger, setting (inpatient/outpatient), pharmaceutical form, commercialized pharmaceutical specialties or available alternatives, and material/personal resources.
Prior to its implementation, e-learning training was carried out for the healthcare professional involved.
What has been achieved?
A total of 25.3% (379/1498) of the pharmaceutical specialties included in PTG were HD. Thirteen HD groups were identified. Due to the fact that in the outpatient setting the drug is dispensed to the patient in its original container, the actions implemented were only carried out for inpatients, representing these 9/13 of described groups. The established training was carried out by the 89% of professionals. Proposed measures for HD are summarized in Table 1.
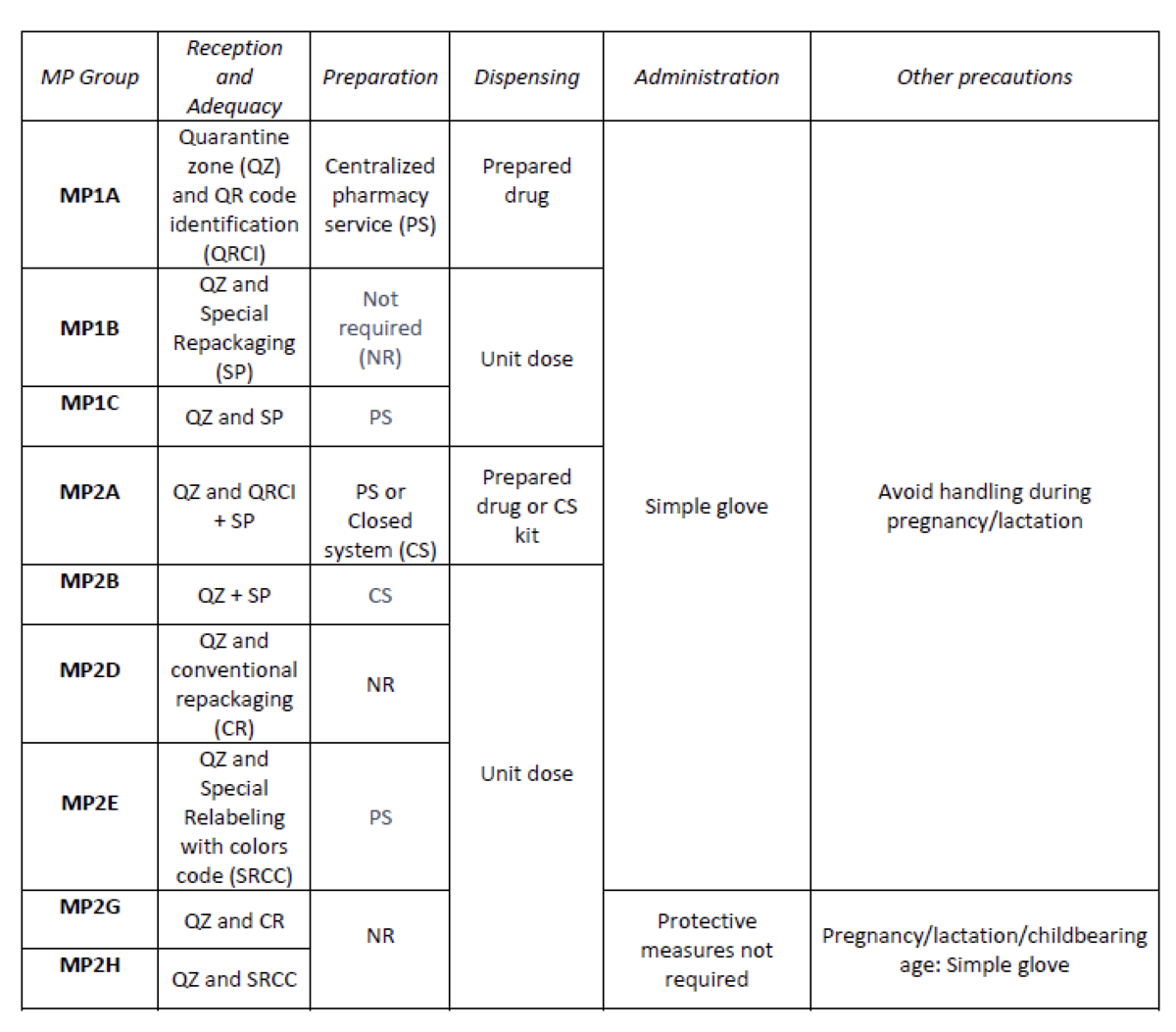
What next?
Monitoring and evaluation of the circuit
Stewardship initiative for COVID-19 monoclonal antibodies led by clinical pharmacists
European Statement
Clinical Pharmacy Services
Author(s)
Petra Rozsívalová, Věra Zdanovcová, Lenka Beková, Martina Maříková, Marcela Heislerová, Vladimír Koblížek, Petr Šmahel
Why was it done?
By implementing systematic monitoring in our institution, CPs ensure appropriate and safe use of unlicensed treatment available under Emergency Use Authorisation. The secondary goal is mandatory data input to national register and patient clinical outcome feedback to physicians and hospital management.
What was done?
It is vital to bring focus on appropriate use of COVID-19 monoclonal antibodies (mAbs) in high-risk population when safety and efficacy data are scarce. A team of clinical pharmacists (CPs) helped to set up process flow, monitor prescribing of mAbs and provide systematic follow-up on time course of symptoms reported by SARS-COV-2 positive outpatients since March 2021.
How was it done?
Ethics committee approval was obtained for monitoring of patients. Consented patient data are extracted from electronic prescribing system and patients score subjective symptoms using questionnaire on days 7, 29 and 90 after the infusion. The prescriber, indication criteria, infusion application day with regards to PCR positivity and symptom onset and vaccination status are recorded.
What has been achieved?
Currently our small dataset includes 59 bamlanivimab and 63 casirivimab/imdevimab patients. On average, mAbs are administered within 2 days of positive PCR test and 5 days of COVID-19 symptoms onset. Our patients report subjective improvement in symptoms by 7 days post infusion. None of our patients clinically deteriorated because of COVID-19. 100 % of mAbs administered are reported in the national database. In October 2021 we achieved that laboratory confirmed positive patients receive text message with infectionist contact to discuss eligibility for mAbs on 24/7 basis. CPs also implemented information leaflets on mAbs for patients with chronic conditions.
What next?
To date, there are no published descriptive data on real-life utilisation of COVID-19 mAbs in terms of patient characteristics, disease outcome and vaccination status. Our initiative shows na excellent opportunity for CPs to enhance timely and rational mAbs use.
























