OPTIMIZATION AND CENTRALIZATION OF THE HANDLING CIRCUIT OF HAZARD DRUGS FROM THE PHARMACY SERVICE
European Statement
Clinical Pharmacy Services
Author(s)
Mireia Iglesias Rodrigo, Júlia Pardo Pastor, Alba Manzaneque Gordon, Cristina Sangrador Pelluz, Núria Meca Casasnovas, Clara Sebastián Carrasco, Fernando Salazar Gonzalez, Gemma Garreta Fontelles, Jordi Nicolás Picó
Why was it done?
Due to the risk posed by the handling of Hazard Drugs (HD) in the healthcare field, it is necessary to implement circuits that guarantee the professional’s safety.
What was done?
Create an internal classification of HD based on the NIOSH List of Hazard Drugs in Healthcare Setting 2020, to optimize the circuit of its handling from its receiving to its administration.
How was it done?
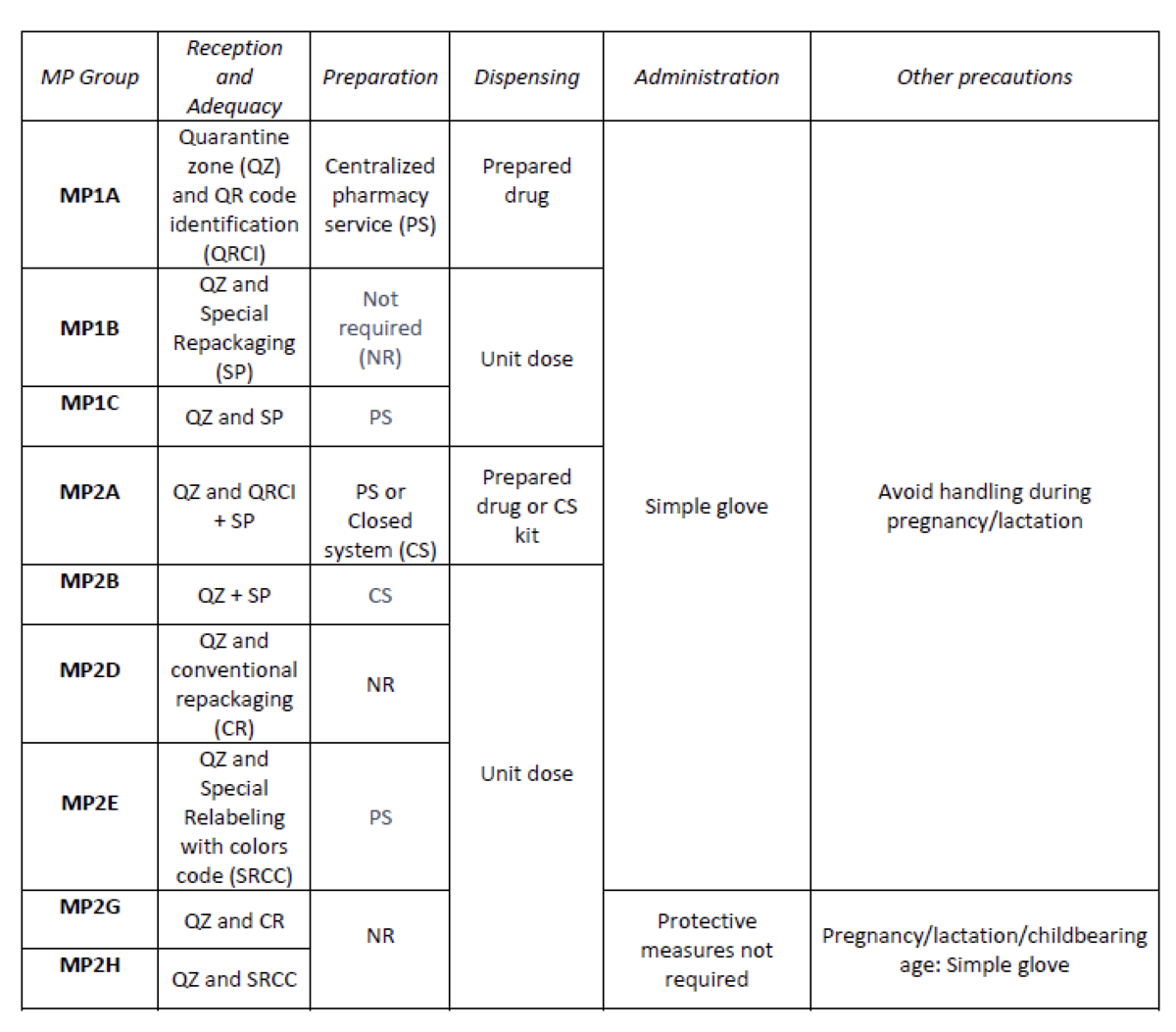
Considering the HD included in the Pharmacotherapeutic Guide (PTG) of our center, the stages of reception and adaptation/preparation/dispensing/administration and other precautions were analyzed.
Categories were established, analyzing the needs of each stage according to: NIOSH level of danger, setting (inpatient/outpatient), pharmaceutical form, commercialized pharmaceutical specialties or available alternatives, and material/personal resources.
Prior to its implementation, e-learning training was carried out for the healthcare professional involved.
What has been achieved?
A total of 25.3% (379/1498) of the pharmaceutical specialties included in PTG were HD. Thirteen HD groups were identified. Due to the fact that in the outpatient setting the drug is dispensed to the patient in its original container, the actions implemented were only carried out for inpatients, representing these 9/13 of described groups. The established training was carried out by the 89% of professionals. Proposed measures for HD are summarized in Table 1.
What next?
Monitoring and evaluation of the circuit
Semi-automatic COVID-19 vaccine preparation for upscaling of vaccination
European Statement
Production and Compounding
Author(s)
Denise van der Nat, Anouk Lindemans, Laurens van Rijn, Wilfred Weijers, Elisabeth Ruijgrok
Why was it done?
COVID-19 is an ongoing worldwide pandemic which causes millions of deaths. To reduce COVID-19 mortality and morbidity, vaccines are developed. However, preparing COVID-19 vaccines before administration is a time consuming process. To accelerate this process and increase efficacy for health care workers, the Vaxtractor was designed in January 2021. We aimed to examine the quality of COVID-19 vaccines prepared with the Vaxtractor.
What was done?
Development and testing of a device (Vaxtractor) for semi-automatic large-scale preparation of COVID-19 vaccines.
How was it done?
With the Vaxtractor, the desired volume of vaccine is drawn up automatically in syringes from two vials of vaccine simultaneously. A sterility test, measurement of accuracy and uniformity of dosage units tests were performed in September and October 2021. For the sterility test, 22 syringes were filled with 0.5 ml Tryptic Soy Broth and these were incubated at 25°C for seven days followed by a seven day incubation period at 30°C. For the accuracy and uniformity of dosage units test, 60 syringes were filled with vaccine. Subsequently, the filled and emptied syringes were weighed. Furthermore, a time analysis was performed on manually and semi-automatically prepared COVID-19 vaccines.
What has been achieved?
The sterility test showed no visual sign of growth of micro-organisms. Furthermore, the weight of 60 prepared Spikevax® vaccines deviated less than 5% compared to the average weight of the vaccines. Also, the observed volume per vaccine deviated less than 5% compared to the declared volume. Besides that, preparing COVID-19 vaccines with the Vaxtractor was about three times faster compared to manually prepared vaccines and reduced the risk of needlestick injuries.
What next?
The Vaxtractor can be used to safely prepare Spikevax® vaccines. In the next months we will assess the quality of preparing Comirnaty® vaccines with the Vaxtractor. If these results are positive, the device can be implemented at large scale at the in- and out-of-hospital setting. This will contribute to effective upscaling of COVID-19 vaccination.
Design and implementation of a course on “Improving the understanding of biosimilar formulation science through Real-World Training”
European Statement
Education and Research
Author(s)
Paola Minghetti, Giuseppe Danilo Norata, Francesca Selmin, Paolo Rocco, Vito Ladisa, Margherita Galassi
Why was it done?
The availability of mAbs to treat different pathologies is steadily growing, causing a steep increase in the level of training needed in different areas of pharmacists’ intervention, including compounding, handling and storage. As this process will be sustained by the increasing availability of biosimilars, pharmacists, the key health professionals responsible for their compounding and handling, will face new challenges.
The aim of this project is to overcome the common problems encountered by hospital pharmacists in obtaining education on biosimilars, included limited financial support, heavy workload or inadequate educational resources.
What was done?
A self-paced educational course has been designed and implemented with the aim of providing a fundamental grounding in the physical chemistry, pharmacology and technology of monoclonal antibodies (mAbs)-based medicines in oncology, both originators and biosimilars and the methodology associated with their compounding and handling.
The primary target audience for this project consists of hospital pharmacists in the EU, though students in the specialization in Hospital Pharmacy and community pharmacists may benefit from the course.
How was it done?
The course has been designed and developed to address, previously assessed, unmet educational needs. The resulting format comprises both theoretical and remote real-world training on the pharmacology, technology and stability of mAbs, the technology and rationale of biosimilars and the regulatory aspects of biotechnological medicinal products.
What has been achieved?
A series of webinars in on demand movie format has been produced. The webinars contain a comprehensive theoretical section – covering biosimilar mAbs pharmacology and formulative and regulatory aspects – and a practice section in which the preparation steps of oncology mAbs are filmed and discussed in a hospital setting. All training activities have been recorded in remote both in Italian and in English. Every module is designed to be used as a single unit and has a duration of approximately 30 minutes. The total duration of the course is 8 hours.
What next?
The course will be CME accredited in Italy through Fondazione Francesco Cannavò, nonprofit CME provider of the Federation of Italian Pharmacists Associations. It will be made available to pharmacists through national and international CME platforms, providing fundamental grounding in the methodology associated with oncology monoclonal antibody biosimilar formulation.
IMPLEMENTATION OF AUTOMATED COMPOUNDING TECHNOLOGY IN A SPANISH HOSPITAL PHARMACY
European Statement
Production and Compounding
Author(s)
CARMEN MARÍA VALENCIA SOTO , ADELA GARCÍA-AVELLO FERNÁNDEZ-CUETO, SARA BARBADILLO VILLANUEVA, MARÍA OCHAGAVÍA SUFRATEGUI, MARÍA VICTORIA VILLACAÑAS PALOMARES, VIRGINIA MARTÍNEZ CALLEJO , MARÍA MARTÍN LÓPEZ, MARÍA RIOJA CARRERA, PAULA DEL RIO ORTEGA, MARTA VALERO DOMÍNGUEZ
Why was it done?
This project aimed to optimize security in the production workflow through automation of anti-cancer drugs compounding.
The use of recognition systems and gravimetric control guarantee traceability and accuracy in the compounding process, therefore improving patient safety.
Robotic systems avoid exposure to cytotoxic drugs, promoting healthcare operator safety. Moreover, once loaded, it runs automatically, liberating the operator for more complex preparations.
What was done?
In 2021, our hospital pharmacy implemented APOTECA platform, including management software (APOTECAmanager), two guided preparation systems for semiautomatic compounding (APOTECAps) and a robotic system for aseptic preparation of antineoplastic drugs (APOTECAchemo).
How was it done?
We configured each drug in the management software: dimensions, density, stability and expiration data, solvent, bags and transfer set information, QR code, etc.
A 3-phases process was scheduled:
– Integration between APOTECA and the hospital’s Electronic Prescribing Software (EPS). Carried out between November and December 2020.
– Training period: 8 weeks between May and July 2021, including pharmacists and technicians with progressively incorporation to real compounding.
– Real production analysis: 8 weeks between July-September 2021 (38 days, excluding weekends and bank holidays). Previously trained staff gradually trained the rest of the personnel.
What has been achieved?
During the 8 weeks considered, 4629 doses were elaborated, excluding clinical trials preparations.
APOTECA production supposed 85% (3944) of our daily compounding: 62,8% (2475) with the 2 semiautomatic systems and 37,2% (1469) with the robot. 99% of the doses prepared in APOTECAchemo were infusion bags and 1% syringes. In APOTECAps, 85% were infusion bags and 15% syringes.
Average dosage error for all preparations was 0,95% (±1,13) for APOTECAchemo and 1,57% (±1,31) for APOTECAps.
Up to data collection, 67 substances that fulfilled the criteria had been processed in APOTECA system and 41 of these in APOTECAchemo.
The top five ingredients compounded in APOTECA were: paclitaxel, carboplatin, pembrolizumab, etoposide and fuorouracil.
What next?
The implementation of this technology has improved patient and operator safety, as well as our daily workflow.
To ensure an optimal use we need to increase robot production by optimizing its operating hours and promoting more preparations in advance.
How to become a resilient chemotherapy preparation unit?
European Statement
Patient Safety and Quality Assurance
Author(s)
Victorine MOUCHEL, Romy LINOSSIER, Chloé FERCOCQ, Jean-Luc PONS, Lucie BAILLET
Why was it done?
Injectable anti-cancer drugs are critical drugs and production disruption would result in discontinuity of care. Moreover, 60% of the production is dedicated to external clients as part of outsourcing contracts. To strengthen client’s confidence, we achieved the ISO 9001 certification in 2019. Implementing a BCMS is part of the overall quality and resilience process.
What was done?
In our hospital centre, production of injectable anti-cancer drugs is centralised in a chemotherapy preparation unit. Within the unit, we decided to implement a business continuity management system (BCMS). Therefore, we established and validated a business continuity plan (BCP) to face a production disruption and continue the delivery of products.
How was it done?
We followed the ISO 22301:2019 standard methodology. First, we performed the risk assessment as described by the ISO 22300: 2018 standard. A multidisciplinary working group (pharmacists, pharmacy technician, quality engineer) identified and analysed the risks likely to threaten the unit’s business continuity (BC). Risks were rated in term of criticality (Cr) from 1 to 4 and risks with Cr ≥ 3 were considered as priority risks. Then, a business impact analysis was led by the pharmacists and validated by the department chief. Strategies were set to face priority risks in accordance with the BC objectives. Finally, we documented the BCP and validated it thanks to tests followed by debriefing.
What has been achieved?
The risk assessment highlighted 23 risks and 13 of them (57%) were rated as priority risks. Most of the risks revolve around unavailability of production equipment or premises (fire, flood, natural disaster). The treatment of 7 of these risks was included in the action plan for 2021. Three strategies were documented to treat these risks: extended opening hours of the unit, closed system transfer device used in a contaminated isolator and production relocation in two other centres. Five tests were conducted to check necessary procedures and devices to use these strategies (closed system transfer device, remote access, transportation). Tests will be repeated yearly to maintain the BCP.
What next?
In conclusion, implementing a BCMS represents a continual improvement approach that will improve the unit’s ability to cope with a crisis in an appropriate way.
How robotics improved safety and working efficiency in a European premium cancer institute
Pdf

European Statement
Production and Compounding
Author(s)
Mathilde Roche, Annabelle Angapin, Vincent Blazy, Alexandre Hyvert, Loretta Moriconi, Matteo Federici, Bintou Diawara, Cindy Monnel, Lison Ferreol, Assia Mitha, Hail Aboudagga, Romain Desmaris
Why was it done?
Initially, robot’s operations required prescription re-transcription and chemotherapy relabelling by technicians, leading to manual data entry risks. Robots are known for high-standardised procedures, great repeatability and limited human intervention: adding bidirectional interface enabled improvement of patient safety. Moreover, it shows significant benefits during the compounding process, streamlining pharmacy workflows and ensuring full and paperless traceability.
What was done?
In 2018, our chemotherapy production unit implemented an automated anticancer drugs compounding platform, embedding two APOTECAchemo robots. This aims to meet the increasing patient-specific chemotherapy demands (78,000 preparations/year). In order to minimise human risk and optimise work efficiency, implementation of a bidirectional interface between the robots and the hospital’s Electronic Prescribing Software (EPS) was considered as mandatory, to allow exchange and clinical information retrieval.
How was it done?
In 2020, pharmacists and the IT team defined the interface specifications. Bidirectional information flow was implemented using Health Level Seven (HL7) standards. Interface between EPS and APOTECAmanager was developed and a comparative robot performance analysis was undertaken by evaluating processed drug products, compounded preparation numbers and actual average usage time per day. The staff (i.e. two technicians) remained identical. Data were retrieved from robot’s embedded statistical tool over three months, before (March-May 2020) and after interface implementation (July-September 2020).
What has been achieved?
During these six months, 13,746 preparations were compounded, with 95% infusion bags and 5% elastomeric pumps. Most of these preparations were produced in advance (administration on day+2 or day+3). After interface implementation, the average production raised by 40.5% (from 1,905 to 2,676/month). Interface implementation increased also the average robot operating hours from 3.6 hours/day/robot to 5.8 hours/day/robot (+61.1%). In total, 19 different molecules were compounded, including conventional anticancer drugs and monoclonal antibodies with the number of reconstituted drug vials increasing by 38.1% (from 625 to 863).
What next?
Interface between robots and the EPS was successfully implemented, thereby enabling improved safety and efficiency. Today, syringes and paediatric preparations are still made manually. They require visual and analytical controls to verify their conformity. Mid 2021, a third robot customized for syringes and paediatric preparations will be installed in the compounding unit, to secure these preparations in a more efficient way.
Aflibercept redosification impact in a second-level hospital.
Pdf

European Statement
Production and Compounding
Author(s)
Javier Alfonso Buendía Moreno, Andrea Portela Sotelo, Lidia Martínez Valdivieso, Jaime Fernandez-Bravo Rodrigo, Gema Marcos Pérez, Dolores Barreda Hernandez
Why was it done?
Aflibercept is an Agent against Vascular Endothelial Growth Factor A (VEGF-A) whose intravitreal indications such as Age-related Macular Degeneration (AMD), Macular Edema (ME), Retinal Vein Occlusion (RVO), have a high economic impact on a Pharmacy Service (PS) budget.
What was done?
A protocol for the redosification of aflibercept intravitreal therapy was implemented by the Commission of Pharmacy and Therapeutics and the Ophthalmology Service, which proposed the redosification of aflibercept vials into sterile syringes for intravitreal use.
How was it done?
Aflibercept 4 mg vials were recompounded by infirmary staff in a horizontal laminar air flow cabinet into syringes with the recommended dosage of 2 mg, hence one vial could approximately be fractionated for the production of 2,5 syringes.
The variables compiled to maintain the trazability of aflibercept through the programmes of computerized clinical history, MambrinoXXI® and electronic prescription, Farmatools®, were: sex, age, indications, number of spent vials and syringes prepared and average number of syringes dispensed per patient. In addition, it was compared the direct estimated cost of the syringes vs. vials to calculate the saving cost.
What has been achieved?
During the year 2019, 305 patients received aflibercept syringes, 172 (56’4%) were male, the average age was 76 years (41-95). Main diagnoses were 145 AMD, 71 ME, 43 diabetic ME and 33 RVO. The total numbers of vials spent were 341, the syringes dispensed were 1174 and the average number of syringes dispensed per patient was 3’85. The total price of one vial was 612’31€, so one redosificated syringe in the PS approximately costs 204’10€. Therefore the use of syringes instead of vials had a potential saving cost of 331.672€ (58’01%) if the vials would have been used. The cost reduction of the intravitreal therapy with aflibercept supposed a saving of 1’58 % of the total expenditure of the PS during 2019.
What next?
The optimization of aflibercept intravitreal therapy is a big cost-effective measure for reducing costs in a PS. It helps to reduce costs in a therapy that is increasing the number of patients each year contributing to the financial sustainability of Health Systems and improves the efficacy of the resources of PS.
Integration of clinical trials management into a safe and fully-automated onco-haematology workflow
Pdf

European Statement
Production and Compounding
Author(s)
FRANCESCA VAGNONI, ANDREA MARINOZZI, SABRINA GUGLIELMI, CHIARA CAPONE, FRANCESCA MURA, ADRIANA POMPILIO, SIMONE LEONI
Why was it done?
The management of CT requires thorough documentary evidence and well-organized reporting system in compliance with the Good Clinical Practice. Since 2009, the entire onco-haematology workflow is fully-controlled by information technology devices and robotic systems to prevent medication errors and guarantee data integrity. The implementation of APOTECAtrial was aimed to extend the same level of control to CTs.
What was done?
In 2018, a clinical trial (CT) managing system (APOTECAtrial) was integrated into the existing fully-automated workflow of the chemotherapy production unit. APOTECAtrial was developed to enable real-time visualization of CT-related data and trace the processing of investigational (IMP) and non-investigational (NIMP) medical products, such as delivery, assignment, preparation, return, and disposal.
How was it done?
A team of hospital pharmacists, physicians, clinical data managers, and IT specialists analysed the CT workflow and defined the system specifications. Data related to IMP/NIMPs (both for parenteral and oral administration), patients enrolled, and investigator/sponsor affiliations were entered into APOTECAtrial and sorted by CT. The onco-haematology unit’s electronic prescribing system was bidirectionally interfaced with APOTECAtrial. Aseptic preparation of patient-specific injectable therapies was implemented in the supporting device for manual preparation that checks dosage accuracy and identity by photographic and barcode recognition.
What has been achieved?
Since 2018, the overall number of CTs managed was 95. In total, 81 IMPs/NIMPs and 135 patients were entered into the system, while 2740 injectable therapies were prepared, 690 oral medications and 60 pre-filled syringes delivered. The following major objectives were achieved: automated inventory accounting and stock management, reduced manual time-consuming activities (i.e. documentation, transcription), standardized reports in digital not-editable format, and full traceability. In addition, audit trail tool tracks all user edits and changes performed at any stages of the CT management by electronically recording user’s name, date, and time. APOTECAtrial was evaluated by clinical research associates (CRA), clinical research organizations (CRO) and CT sponsors and approved for use in the daily clinical practice.
What next?
The project represents a good example of multidisciplinary collaboration focused on improving the quality of the processes in healthcare settings. The implementation of information technology and automation ensures improved data integrity, safety, and working efficiency, which are key determinants for managing CTs in hospital pharmacies.
Standard Operating Procedures for urgent chemotherapy mixture preparation by non-experienced staff
Pdf

European Statement
Production and Compounding
Author(s)
Ana Marín-Romero , Inés Monge-Escartín , Esther Carcelero-San Martín , Gisela Riu-Viladoms , Rubén González-García, Jaume Planas-López , Dolores Merino-Calderón , Rodolfo Juncos-Pereira , Carolina Lesta-Domene , Carmen López-Cabezas, Dolors Soy
Why was it done?
Cytostatics are hazardous drugs that must be prepared under safe and sterile conditions. In some life-threatening situations, there is an urgent need to initiate chemotherapy immediately. However, not all hospitals have experienced personnel in safe-handling cytotoxic drugs for 24 hours and 7 days per week.
The objective is to create a consensual protocol to be used when immediate start of chemotherapy is required, and preparation must be done out of working hours of specialized pharmacy staff. A secondary objective is to confirm that non-experienced staff can prepare cytostatics safely and to guarantee their quality by following this protocol.
What was done?
The oncohematology pharmacy team created a visual guide aimed to pharmacy personnel who do not routinely work with intravenous mixture preparations. This guide includes instructions about parenteral cytotoxic drug preparation for chemotherapy regimens that should be immediately initiated.
How was it done?
Urgent regimens were agreed with clinicians. They are: (i) fixed-dose intrapericardial cisplatin, (ii) intravenous carboplatin and etoposide, (iii) intravenous cisplatin and etoposide, (iv) intravenous cyclophoshamide and (v) fixed-dose intravenous daunorubicin. For schemes with different possible doses, fixed banding doses were agreed with clinicians.
A visual guide with images of all the material and preparation steps (including labelling, packaging and protection measures), for each scheme, was developed and attached to a prescription form to be completed by the physician and associated with a material kit that contained personal protective equipment, expendable material, cytostatic vials and serum bags.
The guide was distributed to pharmacy personnel external to preparation area, accompanied by a training session. Selected trained workers were supervised while preparing the mentioned cytostatic drugs in a simulated-base patient scenario.
What has been achieved?
All the cytostatic drugs were prepared correctly reaching a maximum preparation time of 45 minutes since physician’s prescription. The personnel involved maintained all the specified protection measures and reported feeling confident while cytostatic manipulation.
The guide proved to be useful to cover a possible urgent chemotherapy treatment outside the stipulated work schedule.
What next?
Re-training in safe-handling of cytotoxic drugs should be ongoing with regular updates to ensure a proper follow-up of this guide. This work methodology could be extrapolated to other pharmacy areas with similar needs.
SARS-CoV-2 specimen collection kits: maintaining supply through in-house production
Pdf

European Statement
Production and Compounding
Author(s)
Nikolaus Lindner, Doris Haider
Why was it done?
In Austria, Covid-19 infection rates began to increase in March. At Clinic Favoriten, over 700 patients were treated during the first wave. This resulted in an increasing demand of specimen collection sets. Even though various wholesalers and contractors were contacted, the orders could not be served in a quantitative or timely manner. These circumstances forced the pharmacy to look for alternative solutions.
What was done?
During the first wave of SARS-CoV-2 infections the hospital pharmacy of Clinic Favoriten, Vienna’s specialised Covid-19 center, assembled specimen collection sets manually to meet rising demands, compensate for shortages and secure vital diagnostics supply.
How was it done?
In collaboration with the laboratory department and other clinics of the Vienna health care group appropriate materials with CE-certification were sought to assemble a set that is easy to handle concerning production, distribution and application.
Sterile plastic tubes were filled aseptically with physiologic saline and labelled. Tubes and sterile swabs were then packed in a plastic bag that was sealed with a label providing general instructions for use. Manufacturing protocols as well as batch documentation ensured quality assurance and traceability.
Major obstacles included availability and suitability of the needed materials. Manufacturers of tubes and swabs had to be changed over time, which required close communication with medical wards and the laboratory department.
What has been achieved?
Over a period of seven weeks 2.033 specimen collection sets were assembled. In detail, a total of 20.330 swabs were packed and 10.165 tubes were filled. Through this measure a continuous supply of specimen collection sets, essential for further Covid-19 testing, was secured.
Moreover, the importance of a pharmacy in-house production with the aim of maintaining supply security was acknowledged throughout the entire hospital.
What next?
The initiative has demonstrated that pharmacists play a vital role in handling product shortages and maintaining supply security. In the future, the pharmacy will reinforce to monitor trends even more and will thus be able to balance changing demands and non-availabilities. Like this, the existence of an in-house pharmacy department securing appropriate supply will gain more and more significance. In times of increasing shortages, the initiative serves as a model for other healthcare systems confronted with similar difficulties.

























