OPTIMISATION OF AN IN VITRO RED BLOOD CELL LABELLING PROTOCOL WITH TECHNETIUM-99M FOR THE DETECTION OF GASTROINTESTINAL BLEEDING
European Statement
Production and Compounding
Author(s)
Y Fardo, J Verdier, F Al Shoukr, A Maget, E Verrechia, G Rondelot, S Ben Mahmoud
Why was it done?
An optimisation of an in vitro labelling protocol for red blood cells (RBCs) with technetium-99m (Tc-99m) for the detection of gastrointestinal bleeding was carried out. The initiative aimed to improve a reference protocol that did not achieve the expected labelling yields under local working conditions. By systematically testing several modifications to the protocol parameters, the variables influencing labelling efficiency were identified, and adjusted conditions were defined to obtain satisfactory and reproducible yields.
What was done?
The implementation of existing reference protocols regularly resulted in labelling yields below expected values around 50–60% instead of more than 85%, which limited diagnostic reliability. The objective was to identify the key variables that significantly affected yield, with the aim of optimising the protocol parameters to achieve higher and more consistent results suitable for routine hospital practice
How was it done?
The standard in vitro RBC labelling CNHIM procedure was used as the baseline. Forty-one experimental runs were performed, testing variations in pyrophosphate dilution, centrifugation forces, and agitation times. The resulting labelling yields were recorded and analysed by multiple linear regression to quantify the impact of each parameter on the overall efficiency.
What has been achieved?
Statistical analysis indicated that decreasing both pyrophosphate concentration and centrifugation force during the washing step of unbound Tc-99m significantly improved the yield (+37.0% and +17.2%, respectively). The other tested parameters showed no significant effect. The optimised protocol, diluting reconstituted pyrophosphate at 1:400 and applying a centrifugation force of 700 g during separation steps, consistently achieved labelling yields above 95%.
What next?
This initiative demonstrates that a simplified and reproducible labelling protocol can improve efficiency and reliability in the detection of gastrointestinal bleeding, without increasing procedural complexity. These findings could be easily applied in other hospital laboratories using in vitro RBC labelling.
INFLUENCE OF PACKAGING ON STABILITY: EXAMPLE OF 5% LUGOL’S SOLUTION
European Statement
Production and Compounding
Author(s)
K. Lefèvre (1), M. Ramond (1), A. Bourges (1), E. Gueret (1), S. Vrignaud (1), V. Lebreton (1,2)
(1) Angers University hospital center, Pharmacy Department, Angers, France
(2) MINT Inserm 1066, CNRS 6021, University of Angers, France
Why was it done?
Lugol’s solution 5% (iodine/iodide) is used to saturate the thyroid before MIBG scintigraphy. Due to iodine’s high volatility, the stability of the solution depends heavily on its packaging. Random shelf life quality controls revealed out-of-specification iodine levels, raising concerns about iodine loss linked to poor packaging.
What was done?
This study aimed to evaluate iodine loss over time from 5% Lugol’s solution depending on the type of packaging, before opening, in order to propose improvements ensuring better stability.
How was it done?
Three packaging types were tested: Type I amber glass bottle with dropper and no secondary packaging, the same bottle with a cardboard secondary packaging and the same glass bottle with a white Bakelite screw cap and secondary cardboard packaging.
Iodine content was measured weekly in triplicate for at least three months using an automatic titrator (Mettler Toledo T5) with a redox electrode and 0.1M sodium thiosulfate titrant. Previously method was validated according ICH guidelines (ICH Q2A). Parameters such as accuracy, precision, linearity and LOQ were evaluated. Iodine loss was calculated and modeled over time (mean ± 95% confidence interval) with following equation A=Aoe-kt (k and t expressed in Day (D).
What has been achieved?
After 9 weeks, iodine losses reached 28.5 ± 0.8% (with secondary packaging) and 58.9 ± 0.3% (without), even before opening, for the dropper bottles. The iodine concentration followed a first-order kinetic degradation for all packaging, k = -0.01D-1 for both with dropper and k=0.004 D-1 with bakelite cap. The Lugol’s solution no longer met specifications after just 1 month. In contrast, bottles with Bakelite caps remained stable for up to 6 months, with less than 2% iodine loss.
What next?
Packaging has a critical impact on the stability of 5% Lugol’s solution. To improve preservation, several changes were implemented: bottles are now closed wtih Bakelite caps, and droppers are supplied separately in cardboard secondary packaging. The shelf-life before opening was reduced from 1 year to 6 months and limited to 1 week after opening.
COMPUTER DEVELOPMENT OF THE MAGISTRAL FORMULATION MODULE FOR UNIFICATION OF THE PRODUCTION AREA
Pdf

European Statement
Production and Compounding
Author(s)
A. Henares-López, V. Collados-Arroyo, C. Mayo-López, R. Fernández-Caballero, L. Carrasco-Piernavieja
Why was it done?
A new magistral formulation and mixes management program was developed in the Farmatools® software to optimize the procedures and records of this area.
What was done?
The correct management of compounded formulation is important for the labor of a pharmacy service, ensuring safe preparation and adequate traceability. In our pharmacy service we only had a simple formula registration program that only allowed formulas to be added to the stock but the rest of the work (protocols, batches, preparation staff, validation) was recorded on paper.
How was it done?
Taking advantage of the implementation of the Farmatools® software in our pharmacy service that already had a simple compounding module, it was necessary to carry out a development to add improvements such as registration of raw materials, quarantine states, expiration notices, creation of manufacturing protocols for each preparation, formula programming calendars, improvement of the traceability system (new batch records and other necessary data in the case of biological material), quality controls such as mass uniformity control for capsules, validation section of the formula prepared for the pharmacist, registration of manufacturing staff, improvement of data present on the manufacturing label.
This work was possible thanks to the help of the farmatools® IT staff with whom we held regular meetings.
What has been achieved?
A correct stock of the amount of real raw materials existing in the pharmacy was achieved, complete computerization of the registry of preparations of magistral formulation, including batches, expiration dates, quarantines and necessary quality control records, creation of an electronic recipe book, approval or rejection of elaborate formulas and eliminating any paper records.
What next?
There are still new developments to be implemented, such as the creation of a calendar of scheduled work and a list that allows viewing the preparations pending validation by the pharmacist. Finally, it would be necessary to implement tablets that contain work protocols to avoid the use of paper.
With this program it has been possible to unify the work of the production area in a single program, which results in an improvement in traceability and fluidity in the work.
Analysis of quality indicators in the compounding area through a mobile application
Pdf

European Statement
Patient Safety and Quality Assurance
Why was it done?
To identify improvement opportunities in the Pharmacy Compounding Area through the analysis of indicators obtained from a traceability App.
What was done?
In 2022, a mobile application (App) was designed and implemented to facilitate the traceability of preparations compounded in the Pharmacy (parenteral nutrition, chemotherapy and other individualized sterile preparations). In addition, the analysis of data registered in the App has provided valuable information about the compounding unit performance.
How was it done?
Monthly reports from May to December 2022 were analysed, focusing only on chemotherapy preparations. The indicators selected were: the average number of monthly preparations, weekly workload distribution, daytime distribution of compounded preparations, preparations returned to the Pharmacy, percentage of treatments prepared on the same day of administration, and percentage of preparations compounded after the scheduled administration time.
What has been achieved?
An average of 139 chemotherapy preparations per day was recorded. The daily distribution highlights that Thursdays and Fridays are the busiest days with the 45% of the total weekly preparations. Furthermore, the morning shift carries out most of the compounding work, with 79% of the preparations being compounded before 3 PM. This information might be useful to the management team to better distribute tasks and resources. Data analysis indicates that 62% of the preparations are compounded in advance, while the remaining 38% are prepared on the same day of administration, which is also valuable information to organize the compounding workflow. On average, 59 preparations were returned per month. Finally, we found that 8.1% of the chemotherapy drugs were prepared with a median delay of 47 minutes from the expected time of administration. All these items are currently being monitored as quality indicators in order to find the way to minimize them.
What next?
The analysis of data recorded in the App provides us valuable management indicators for organizing work in the preparation area.
Tracking these indicators serves as a quality tool for the area and helps us identify opportunities for improvement.
How we picked drugs for our automated preparation
Pdf

European Statement
Production and Compounding
Author(s)
Teimori Kaveh, Lunnan Asbjørn , Komnenic Aleksandar, Gleditsch Espen, Duedahl Hende Camilla
Why was it done?
Oslo Hospital Pharmacy is working to standardize and automate 10% of Oslo University Hospital’s annual consumption of two million parenteral medication doses. They aim to provide 200,000 ready-to-administer doses to OUS, starting with a trial in 2025 and scaling up to 200,000 doses by 2028. This initiative addresses efficiency, reduces nurse workload, and minimizes medication errors, addressing healthcare workforce challenges and ensuring timely and accurate medication delivery at Oslo University Hospital.
What was done?
Drugs were selected for inclusion in implementation of automated preparation of ready-to-use syringes and bags.
How was it done?
Oslo Hospital Pharmacy is dedicated to providing market-competitive ready-to-administer medications through a flexible selection process. This process involved a thorough analysis of parenteral medication usage in five reference care units over eight months. We compared consumption in these units (69 beds) to the entire hospital (2,031 beds) to align with Oslo University Hospital’s needs. Collaborations with international partners in the Netherlands and Denmark confirmed shared priorities, especially in ready-to-administer antibiotics, validating their meticulous selection process. Oslo Hospital Pharmacy’s strategy underscores their commitment to addressing healthcare challenges effectively with global validation.
What has been achieved?
The following 12 medications were selected for the initiative: Piperacillin/tazobactam 4g, Ampicillin 2g, Vancomycin 1g, Vancomycin 0.5g, Cefotaxim 2g, Cloxacillin 2g, Cefazolin 2g, Propofol 10 mg/ml, Fentanyl 50 microg/ml, Ketamin 10 mg/ml, Benzylpenicillin 3g and Benzylpenicillin 1.2g.
Results showed that the reference care units consumed 14 ampoules or vials per bed, while Oslo University Hospital consumed 80, suggesting a representative and potentially even larger demand across the hospital.
What next?
The established drug selection procedure offers an organized method for incorporating new medications. This well-defined medication list facilitates the selection of the most appropriate automation system for implementation. Considering the prevalent staff and medication shortages on a global scale, many institutions are increasingly considering the adoption of automation in their drug preparation departments. We aspire that our method can offer valuable assistance in their pursuit.
Development of standard kits with utensils for outpatient parenteral antibiotic therapy
Pdf

European Statement
Production and Compounding
Author(s)
Louise Rasmussen Duckert, Marianne Kjettrup Jensen, Mette Lethan, Trine Schnor
Why was it done?
The hospital pharmacy wishes to support the implementation of OPAT and during the process the need for standardised kits with utensils was identified. The availability of kits with necessary utensils for aseptic handling of parenteral infusion would simplify and standardise the work for hospital and home nurses. Considerations regarding patient safety and sustainability were also in favour of the kits, as choice of utensils could secure compliance to regional guidelines considering use of closed systems and rinse of the line after infusion. Kits containing the exact needed utensils for an administration also reduces the possible waste.
What was done?
The hospital pharmacy has composed standard kits with utensils for outpatient parenteral antibiotic therapy (OPAT).
How was it done?
The kit is composed in collaboration between the pharmacy, hospital nurses and home nurses. The best suited infusion set was chosen – a closed system with two spikes for antibiotic mixing and infusion. Hereby nurses avoid direct contact with antibiotics and avoid antibiotic aerosols in the citizen’s home. The infusion set contains no PVC, phthalates or latex. When fully emptied the infusion set can be discarded as regular waste.
The kit also contains a sterile cover for the workstation, sterile ethanol swabs, gloves, pre-filled saline syringes for rinse of the line after infusion and a written manual. All is packed and labelled by the hospital pharmacy and lot numbers are registered for traceability.
What has been achieved?
The kits have been tested in selected municipalities and the content of the kit has been adjusted. As a result of the feedback a film has been recorded showing the handling of the infusion set. The video is used for training and a QR code on the written manual guides the home nurse to the video if needed. The kit is now used widely in the region and response is positive. With the set-up being identical in all municipalities in the region, handling antibiotics and utensils is simpler for the hospital nurse at discharge.
What next?
As the number of patients in home-based OPAT rises, experiences with the kits will probably result in wishes for adjustments. A new kit with utensils for changing PVK is under development.
DEVELOPMENT OF A PATIENT-CONTROLLED ANALGESIC MIXTURE FOR POSTOPERATIVE PAIN CONTROL
European Statement
Production and Compounding
Author(s)
María Molinero, Virginia Puebla, Cristina González, Lidia Ybáñez, Gonzalo Hernando, Natalia Sánchez-Ocaña, Javier Corazón, María de la Torre, Jose Manuel Martínez
Why was it done?
This technique provides autonomy to the patient allowing to adjust the dose based on the intensity of pain. It has been demonstrated that small on-demand doses of analgesia provide a reduction in the final dose, thus reducing side effects. In addition, by minimizing the possible delay in the administration of analgesia, the anxiety associated with pain and exacerbations is reduced.
What was done?
Hospital Pharmacy Service in collaboration with Acute Pain Unit has developed a protocol for an analgesic mixture for intravenous administration in continuous infusion based on tramadol, dexketoprofen and haloperidol. It is a patient-controlled analgesia (PCA) administered by pump for the treatment of acute postoperative pain.
How was it done?
We performed a bibliographic search of stability studies in order to standardize the analgesic mixture, guaranteeing its physical-chemical and microbiological stability.
What has been achieved?
A mixture of 600mg tramadol, 300mg dexketoprofen and 5mg haloperidol was prepared and it was filtered through a 5-micron filter. It was diluted in 100mL of 0.9% sodium chloride, obtaining a mixture of 125mL. It was sealed and bagged in a photoprotective bag. After the bibliographic search on stability data and physical-chemical compatibility of the mixture, a stability of 14 days at 2-8 ºC was established. Once elaborated, quality control was performed by gravimetry. It was dispensed weekly by stock to the post-anesthesia resuscitation unit. The established perfusion rate is 1.3 mL/h or 1.7 mL/h for 48h. With each rescue, 8mg of tramadol and 4mg of dexketoprofen are released per hour or 4mg and 2mg every 30min, respectively. The maximum dose that can be administered is 400mg tramadol, 150mg dexketoprofen and 2mg haloperidol, except if the patient weighs less than 50kg: 8mg/kg tramadol. If renal insufficiency, dose adjustment was mandatory.
What next?
The centralization of the preparation of intravenous admixtures from the pharmacy service allow us to adjust the expiry date based on stability studies reported in the literature, to maintain the asepsis of the mixture as it is prepared in horizontal laminar flow cabinets, to increase the safety and to secure the traceability.
Critical points in the management of intratumoral treatments in oncology clinical trials
European Statement
Clinical Pharmacy Services
Author(s)
Lorena Garcia Basas, Pablo Latorre Garcia, Eugenia Serramontmany Morante, Patricia Garcia Ortega, Pilar Rovira Torres, Laura Maños Pujol, Isabel Cidoncha Muñoz, Maria Queralt Gorgas Torner
Why was it done?
Increasing number of CT with IT, in different pathologies, with different tumor locations, contributes an increase in the complexity of drug compounding and procedures. Their preparation, administration and handling requirements differ from current therapies.
What was done?
Identification of critical points concerning intratumoral treatments (IT) preparation in patients with cancer included in clinical trials (CT).
How was it done?
Ongoing CT with IT in our unit were reviewed to identify critical points regarding prescription and preparation process. 14 trials with IT, 8 (57%) of which have ongoing patients were identified. Two of these trials are “first in human”. The critical points were:
- Nature of the IT: virus (4, 29%), nanoparticles (3, 21%), ribonucleic acid (2, 14%), cyclic dinucleotides (2, 14%), monosaccharides (1, 7%), phospholipids (1, 7%) and proteins (1,7%).Particularly, virus have special safety measures and transport conditions
- Dosing units: mcg (4, 29%), plaque-forming unit/mL (PFU/mL)(3, 21%), mL (3, 21%), mg (2 14%), ng (1, 7%), 50% Tissue Culture Infectious Dose (TCID50)(1, 7%).
- Prior dilution before filling the syringe: 8 (57%) of our preparations require at least one prior dilution.
- Drug volume to prepare according to the tumor size: 8 (57%) IT preparations depend on the tumor size.
- Depending on the depth of the target tumor lesion (visceral or superficial), different size of needle is required. This is important because different priming volumes of the needles are necessary.
What has been achieved?
The whole information necessary for a complete prescription, validation and correct preparation goes further than information usually needed for current therapies such as chemotherapy. The results of the study of the critical points allow us to elaborate the standardized operational procedures (SOP) for each CT and IT. These SOPs include the necessary information for a correct preparation for each IT, reducing risk of mistakes and achieving uniformity in the process.
What next?
These types of therapies represent a challenge, and pharmacists have an important role in developing new procedures. Communication between radiology, oncology and pharmacy departments in a multidisciplinary teamwork is essential. This information may be useful to other centers due to the lack of experience and SOPs to work with this type of therapy.
OPTIMIZATION AND CENTRALIZATION OF THE HANDLING CIRCUIT OF HAZARD DRUGS FROM THE PHARMACY SERVICE
European Statement
Clinical Pharmacy Services
Author(s)
Mireia Iglesias Rodrigo, Júlia Pardo Pastor, Alba Manzaneque Gordon, Cristina Sangrador Pelluz, Núria Meca Casasnovas, Clara Sebastián Carrasco, Fernando Salazar Gonzalez, Gemma Garreta Fontelles, Jordi Nicolás Picó
Why was it done?
Due to the risk posed by the handling of Hazard Drugs (HD) in the healthcare field, it is necessary to implement circuits that guarantee the professional’s safety.
What was done?
Create an internal classification of HD based on the NIOSH List of Hazard Drugs in Healthcare Setting 2020, to optimize the circuit of its handling from its receiving to its administration.
How was it done?
Considering the HD included in the Pharmacotherapeutic Guide (PTG) of our center, the stages of reception and adaptation/preparation/dispensing/administration and other precautions were analyzed.
Categories were established, analyzing the needs of each stage according to: NIOSH level of danger, setting (inpatient/outpatient), pharmaceutical form, commercialized pharmaceutical specialties or available alternatives, and material/personal resources.
Prior to its implementation, e-learning training was carried out for the healthcare professional involved.
What has been achieved?
A total of 25.3% (379/1498) of the pharmaceutical specialties included in PTG were HD. Thirteen HD groups were identified. Due to the fact that in the outpatient setting the drug is dispensed to the patient in its original container, the actions implemented were only carried out for inpatients, representing these 9/13 of described groups. The established training was carried out by the 89% of professionals. Proposed measures for HD are summarized in Table 1.
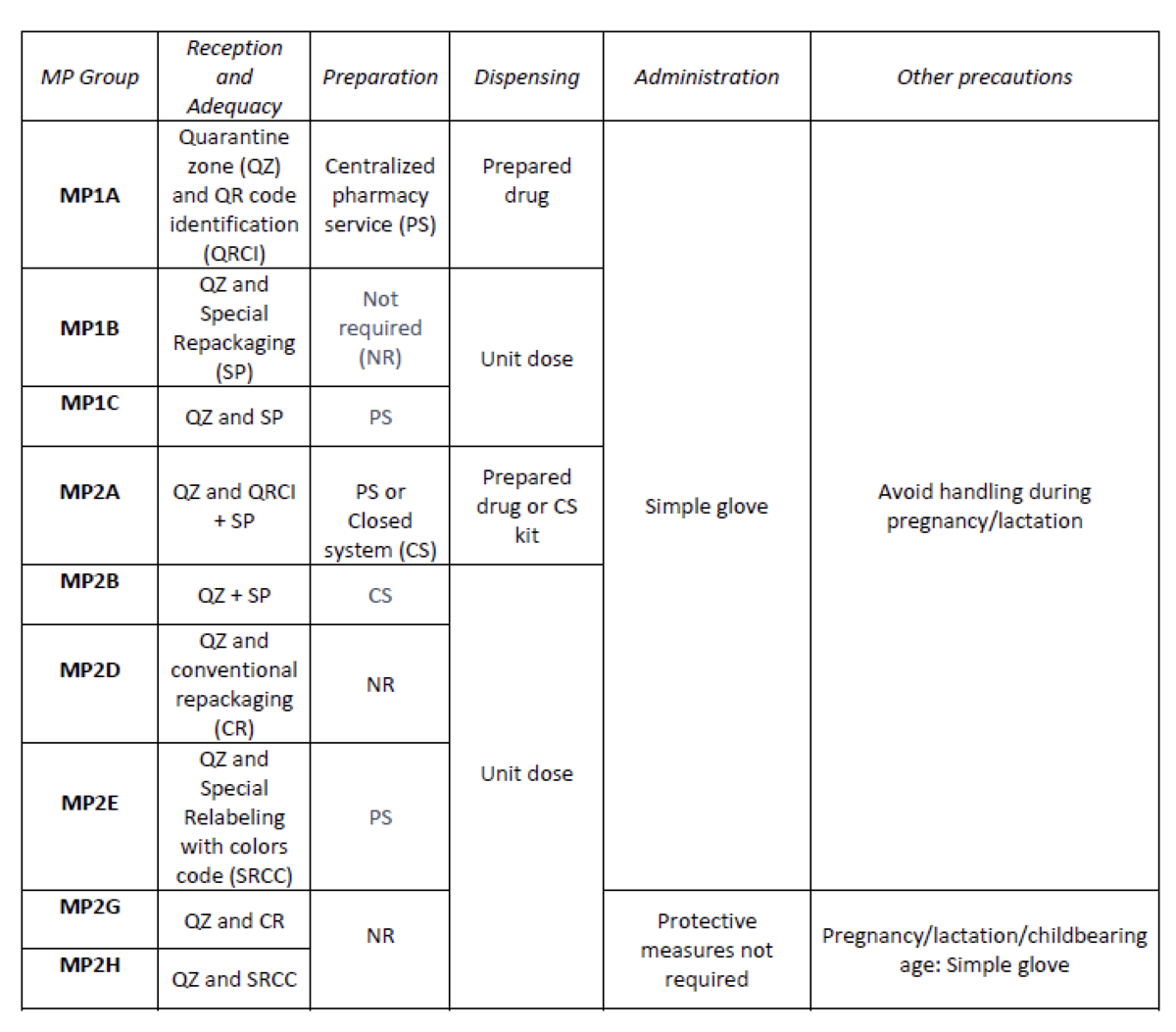
What next?
Monitoring and evaluation of the circuit
Semi-automatic COVID-19 vaccine preparation for upscaling of vaccination
European Statement
Production and Compounding
Author(s)
Denise van der Nat, Anouk Lindemans, Laurens van Rijn, Wilfred Weijers, Elisabeth Ruijgrok
Why was it done?
COVID-19 is an ongoing worldwide pandemic which causes millions of deaths. To reduce COVID-19 mortality and morbidity, vaccines are developed. However, preparing COVID-19 vaccines before administration is a time consuming process. To accelerate this process and increase efficacy for health care workers, the Vaxtractor was designed in January 2021. We aimed to examine the quality of COVID-19 vaccines prepared with the Vaxtractor.
What was done?
Development and testing of a device (Vaxtractor) for semi-automatic large-scale preparation of COVID-19 vaccines.
How was it done?
With the Vaxtractor, the desired volume of vaccine is drawn up automatically in syringes from two vials of vaccine simultaneously. A sterility test, measurement of accuracy and uniformity of dosage units tests were performed in September and October 2021. For the sterility test, 22 syringes were filled with 0.5 ml Tryptic Soy Broth and these were incubated at 25°C for seven days followed by a seven day incubation period at 30°C. For the accuracy and uniformity of dosage units test, 60 syringes were filled with vaccine. Subsequently, the filled and emptied syringes were weighed. Furthermore, a time analysis was performed on manually and semi-automatically prepared COVID-19 vaccines.
What has been achieved?
The sterility test showed no visual sign of growth of micro-organisms. Furthermore, the weight of 60 prepared Spikevax® vaccines deviated less than 5% compared to the average weight of the vaccines. Also, the observed volume per vaccine deviated less than 5% compared to the declared volume. Besides that, preparing COVID-19 vaccines with the Vaxtractor was about three times faster compared to manually prepared vaccines and reduced the risk of needlestick injuries.
What next?
The Vaxtractor can be used to safely prepare Spikevax® vaccines. In the next months we will assess the quality of preparing Comirnaty® vaccines with the Vaxtractor. If these results are positive, the device can be implemented at large scale at the in- and out-of-hospital setting. This will contribute to effective upscaling of COVID-19 vaccination.

























