Centralised DataMatrix reading for drug authenticity verification
European Statement
Selection, Procurement and Distribution
Author(s)
Leonor Romero, Paloma Lozano, Veronica Canales, Vanesa Dechado, Marta Puebla, Ricardo Villarubia, Isamar Gomez, Estefanía Ramírez, Juan Rodríguez, Belén Soto
Why was it done?
According to Directive 2011/62/EU, one of the measures is the inclusion in the secondary packaging of the drug of a Unique Identifier that allows the recognition of a unitary case at any point in the supply chain until it is dispensed to the patient. Another is the development of a European repository that allows the traceability of medicinal products for human use within the European market. In Spain, in accordance with Article 84.1 of Royal Decree 717/2019 of 5 December 2009, the SNSFarma Node was established as an instrument for technological integration and information exchange with the national repository known as the SEVeM.
What was done?
The logistics company of our hospital aggregates several codes corresponding to the Datamatrix of the individual containers in an electronic file, in order to send the reading automatically to Spanish Medicines Verification System (SEVeM).
How was it done?
The shipment of the drugs and the electronic file will be linked by the Seria Shipping Container Code (SSCC), which will univocally guarantee traceability between the two. The Pharmacy Service staff receive the delivery notes by reading the barcode without the need to scan the Datamatrix of each container.
Since the implementation of this project between July 2023 to September 2023, a total of 61 delivery notes have been registered under the code aggregation system with 27 suppliers involved. The number of packages read was 2151.
What has been achieved?
This project ensures the automatic sending of readings to SEVeM and to facilitate the reception of delivery notes at the Pharmacy Services by barcode reading.
This has allowed pharmacy staff to save time in receiving delivery notes, to improve traceability of batches and expiry dates of medicines, to improve stock control thanks to the confirmation of quantities received and to verify the medicines in accordance with European regulations to fight medicine falsifications and ensure that medicines are safe and that the trade in medicines is rigorously controlled.
What next?
A limitation is the existence of suppliers that are not involved in this project since their delivery is not done through the logistics company. In these cases, the datamatrix reading must be performed on each container individually.
Active Pharmacovigilance of COVID-19 vaccines
European Statement
Patient Safety and Quality Assurance
Author(s)
Manuela de Sousa, Ana Catarina Felismino, Ana Rita Pereira, Ivone Máximo, Liliana Pedro, Natacha Santos, Paula Campos
Why was it done?
Vaccines against COVID-19 are classified as “Medicines subject to additional monitoring” by the European Medicines Agency, making it essential to implement active pharmacovigilance systems that allow for the rapid identification of new safety information.
What was done?
Active surveillance of the COVID-19 vaccination process of health-care professionals and immunoallergy patients of our hospital.
How was it done?
By proposal of the Pharmacy Service, a multidisciplinary Pharmacovigilance Committee composed of two Pharmacists, two Physicians and two Nurses was created. The vaccinated professionals and patients were periodically identified, with the support of the Occupational Health and Immunoallergy Departments. Questionnaires to identify Adverse Drug Reactions (ADR) were created in Google Forms® for each COVID-19 vaccine brand and sent to health-care professionals´ institutional email address, or to the email patients provided in the signed informed consent. The responses were exported to an EXCEL® database, analyzed by the Pharmacovigilance Committee, the ADRs identified and communicated to the regional Pharmacovigilance Centre.
What has been achieved?
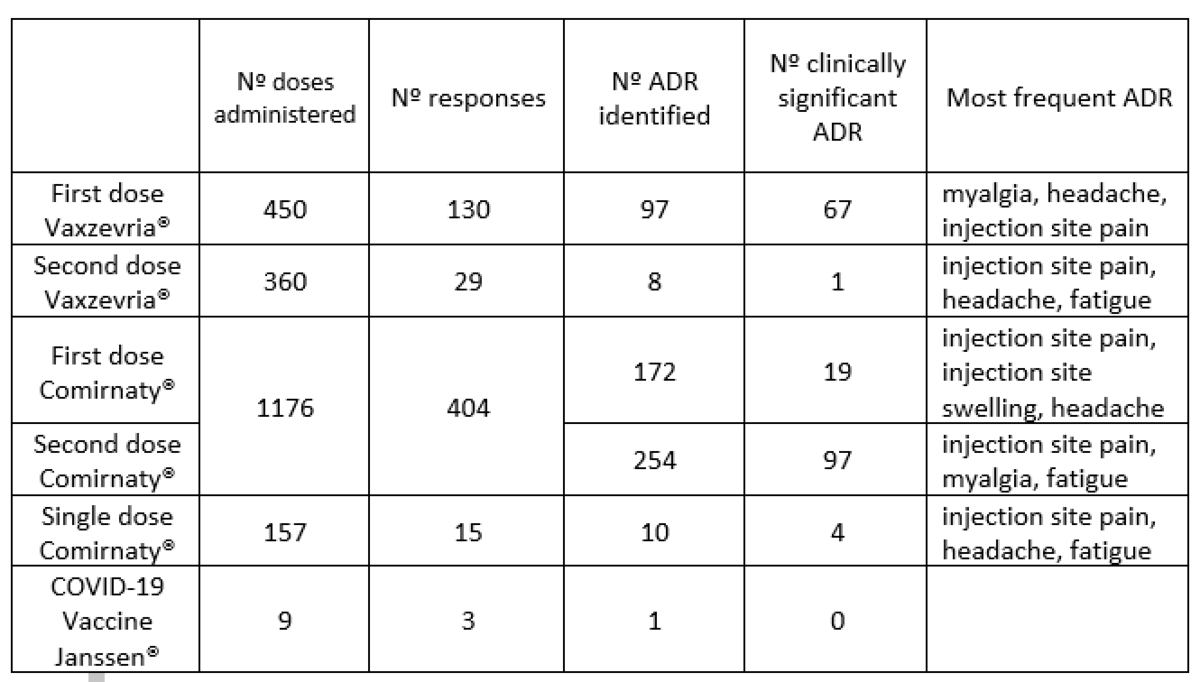
From 29 December 2020 to 31 August 2021 a total of 2141 questionnaires were sent, 578 responses were obtained and 542 ADRs were communicated to the regional Pharmacovigilance Centre. No anaphylactic reactions were reported. Nº doses administered Nº responses Nº ADR identified Nº clinically significant ADR Most frequent ADR First dose Vaxzevria® 450 130 97 67 myalgia, headache, injection site pain Second dose Vaxzevria® 360 29 8 1 injection site pain, headache, fatigue First dose Comirnaty® 1176 404 172 19 injection site pain, injection site swelling, headache Second dose Comirnaty® 1176 404 254 97 injection site pain, myalgia, fatigue Single dose Comirnaty® 157 15 10 4 injection site pain, headache, fatigue COVID-19 Vaccine Janssen® 9 3 1 0
What next?
Increase awareness of the importance of Pharmacovigilance amongst hospital health-care professionals to combat sub-notification of ADRs. We also plan to extend this active Pharmacovigilance program to other medicines in use at our hospital.
A pharmacist-led pharmacovigilance initiative for the first Austrian Covid-19-vaccination campaign
European Statement
Patient Safety and Quality Assurance
Author(s)
Nikolaus Lindner, Katharina Heitzeneder, Nora Hummer, Elisenda Pichler, Doris Haider
Why was it done?
Due to the lack of long-term safety data, the principal goal was to assure a safe and effective use of the available vaccines by coordinating stringent logistical operating procedures as well as by facilitating early detection and evaluation of possible safety signals. A further objective of this initiative was to increase the awareness among healthcare workers regarding the possible health risks associated with a SARS-CoV-2 infection and Covid-19 vaccines as preventable countermeasures.
What was done?
A Covid-19 vaccination pharmacovigilance campaign was implemented in a clinical setting with the focus on patient safety and quality assurance as part of an employee vaccination rollout. The pharmacy department set up a pharmacovigilance service-point to assess vaccine safety as well as potential adverse events and assure patient care by close follow up.
How was it done?
Assessing and reacting to individual safety signals on time represented a critical challenge. Pharmacists designed questionnaires capturing possible adverse events. In order to lower the barriers for participation it was decided to take a paper-based approach instead of electronic distribution. A pharmacovigilance service-point was continuously managed by two pharmacists directly at the vaccination site to achieve a high response rate. Throughout the campaign the completed questionnaires were simultaneously evaluated, as rapid action was key to detect safety signals early and implement measures accordingly.
What has been achieved?
The results showed a high response rate to the questionnaire of 95% and 53% after the first and second dose, respectively. A significant increase of symptoms after the second dose compared to the first dose reflected the findings of the marketing authorisation study. Based on the analysis no further safety precautions were needed. However, appointments before night or weekend shifts had to be discouraged as well as the vaccination of the whole staff from one department on the same day. As a result, disruptions to patient care could be avoided successfully.
What next?
This initiative serves as a valuable model for upcoming vaccination campaigns and especially for pharmacovigilance projects aiming to assess adverse events of recently approved medicines. Moreover, the successfully implemented multi-disciplinary approach represents the basis for further hospital-wide pharmacy projects and may facilitate the implementation of pharmacist-provided vaccination services.

























