Revolutionising pharmacy recognition: evolution of the Australian and New Zealand College of Advanced Pharmacy
Pdf

European Statement
Education and Research
Author(s)
Tom Simpson, Kristin Michaels, Kylee Hayward, Nick Sharp-Paul
Why was it done?
The need to establish a recognition framework that resonated with pharmacists, aligned with their career journeys, and held tangible benefits prompted the inception of ANZCAP. Recognising that existing programmes lacked broad appeal, ANZCAP aimed to redefine recognition in a way that was meaningful, inclusive, and motivated pharmacists towards continuous development.
What was done?
The Australian and New Zealand College of Advanced Pharmacy (ANZCAP) represents a pioneering advancement in pharmacy recognition and career progression. Addressing the limitations of previous models that struggled to gain broad support, ANZCAP emerged as a strategic response to bridge the recognition gap within the pharmacy profession.
How was it done?
The development of ANZCAP commenced with the acquisition of the Advancing Practice (AP) credentialing programme by the Society of Hospital Pharmacists of Australia (SHPA). Previous efforts to engage pharmacists with the programme were reassessed, and a comprehensive review process was initiated to devise an innovative and pragmatic model of recognition. Development comprised multiple phases, including qualitative surveys, workshops, focus groups, and expert consultations. An iterative approach was adopted to refine the model, culminating in a prospective, merit-based system that recognises specialty areas and levels of practice. The focus shifted from individual competencies to broader domains within the National Competency Standards Framework for Pharmacists in Australia 2016, fostering flexibility and practicality.
What has been achieved?
ANZCAP has already recognised pharmacists at all levels – Resident, Registrar, and Consultant – through a Prior Professional Experience process. The college also extends its reach globally, welcoming international pharmacists to join its transformative community.
What next?
ANZCAP’s future involves strengthening the alignment of recognition with promotion and remuneration mechanisms, enhancing engagement among pharmacists. By seamlessly integrating learning experiences with Continuing Professional Development (CPD) activities, ANZCAP aims to foster a culture of lifelong learning and advancement. In the broader landscape, ANZCAP’s journey involves cultivating partnerships with international pharmacy associations, leveraging collective expertise, and fostering an inclusive recognition culture. The programme’s evolution will be guided by feedback, research, and a commitment to advancing pharmacy practice globally.
THE RISK MANAGEMENT OF THE PHARMACY PREPARATIONS IN THE HOSPITAL PHARMACIES (submitted in 2019)
Pdf

European Statement
Production and Compounding
Author(s)
ADRIANA DURCANSKA
Why was it done?
The quality and safety standards of pharmacy preparations are not harmonised throughout Europe. They fall under the national competencies of individual European countries.
What was done?
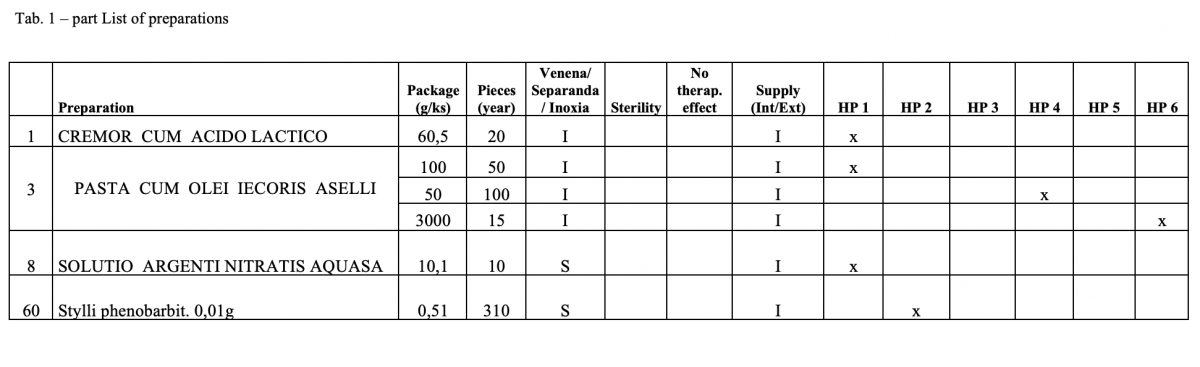
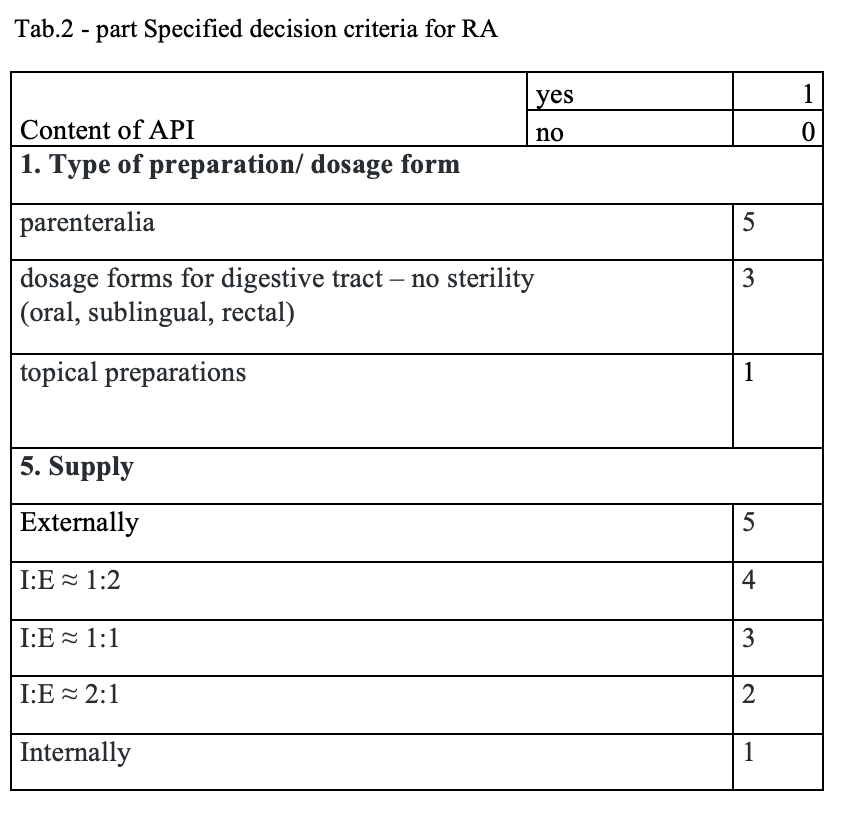
The quantitative risk assessment of the pharmacy preparations for stock in hospital pharmacies (HPs) in accordance with Resolution EDQM CM / Res (2016) 1; to specify the decision criteria for the risk assessment; the risk management of the pharmacy preparations for stock in the country; to design a check list of the risk assessment for extempore preparations.
How was it done?
Out of the total number of 53 hospital pharmacies contacted, 5 pharmacies sent a suitable file.
What has been achieved?
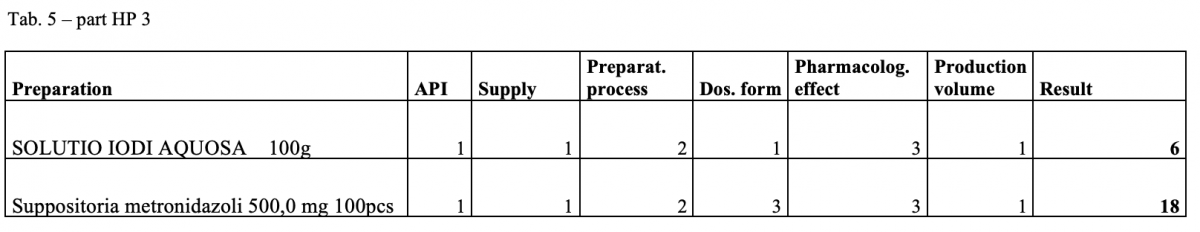
A total of 170 types of medicines are being prepared in HPs. One HP had the result of the risk ≥ 100 when preparing ophthalmic medicines. Annex A is a check list designed to assess the risk of extempore preparations.
What next?
The management is and will be forced to consider its introduction or to use another model: hospital – GMP / outsourcing / central pharmacy preparing and distributing. The aim of using the document in hospital pharmacies of the country.
COMPENDIUM OF POST-GRADUATE ITALIAN HOSPITAL PHARMACY SCHOOLS: AN INFORMATIONAL GUIDE OF ReNaSFO ASSOCIATION – NATIONAL NETWORK OF ITALIAN HOSPITAL PHARMACY SCHOOL STUDENTS (submitted in 2019)
Pdf

European Statement
Education and Research
Author(s)
ANTONIO PIRRONE, FEDERICA MILANI, LUCA CANCANELLI, VALENTINA MARINI , DANIELE MENGATO , ROBERTO LANGELLA , NICOLA REALDON
Why was it done?
On October 5, 2017 the National Network of Italian Hospital Pharmacy School Students (ReNaSFO) was born with the aim to face the various critical aspects of post-graduate Hospital Pharmacy School (SHP), such as the need to make the different paths homogenous among regional SHPs, improve dialogue between colleagues and encourage a more informed approach focused to the training pathway for specialisation. In particular, little official information is available and hard to find about the different realities present in Italy.
What was done?
“Compendium” project is designed to fill this lack and to gather information on post-graduate SHPs operating in Italy. In addition to outlining a summary description of the SHPs, the Compendium is configured as an official tool to respond and provide targeted information to near-graduates and graduates in Pharmacy (who often contact ReNaSFO) interested to approach the SHPs path.
How was it done?
Two project coordinators prepared a list of items submitted to representative ReNaSFO student in every 21 operating SHPs. The items refer to: available places and admission requirements, type of entry test, organisation of didactic lessons, exams and residency training, health facilities affiliated with SHP, potential availability of scholarships, useful links of the SHP or university. The help of universities was fundamental, in particular the helpfulness of SHP directors to collaborate with students.
What has been achieved?
As many as 18 SHPs out of 21 (85.71%) have joined the project: Bari, Bologna, Catania, Catanzaro, Camerino, Genoa, Florence, Milan, Modena and Reggio Emilia, Messina, Naples, Padua, Parma, Pisa, Rome, Siena, Turin and Sassari; of these, 14 schools have already sent their finished “Compendium” form.
What next?
Thanks to the widespread presence of associated ReNaSFO students, the initiative has immediately found interest and participation, reconfirming once again the active and unconditional collaboration between SHP students throughout Italy. Despite a heterogeneous situation between different SHPs, we keep working together hopeful to achieve national uniformity of SHPs and to improve educational objectives and training pathways.
eLearning Environment for Ensuring the Competence in Pharmacotherapy
Pdf

European Statement
Education and Research
Author(s)
Susanna Saano, Tiina Koskinen, Hillevi Rautiainen, Minna Taam-Ukkonen
A NATIONALLY COORDINATED APPROACH TO DEVELOPING HOSPITAL PHARMACY SERVICES IN DENMARK
European Statement
Introductory Statements and Governance
Why was it done?
Approximately 450 people work within clinical and ward pharmacy in hospitals in Denmark. Despite Denmark being a relatively small country, these services have developed at differing paces, and sometimes in different directions. The initiative was set up to coordinate development and innovation in this field, across the whole country.
What was done?
A national group was established to coordinate and develop clinical and ward pharmacy services throughout Denmark. The working group consists of pharmacists and pharmaconomists representing the eight hospital pharmacies in Denmark.
How was it done?
In 2012/2013 fifteen people representing pharmacists and pharmaconomists from the five Danish regions were selected to the working group. These people were typically known to be experienced drivers of innovation and development in the field of clinical and ward pharmacy. The working group meets quarterly and additional work is carried out between meetings. There are no extra resources available to members of the group or their workplaces.
What has been achieved?
The group has produced and implemented minimum standards for ward pharmacy across Denmark. Benchmarking has been carried out using these standards and the baseline has been set. Progress will be measured regularly.
New standards for how often medicine shelf-life checks should be carried out on wards have been developed, resulting in the task being carried out less frequently on most wards, thus releasing resources to more clinically related tasks, at a time where extra resources are scarce.
Two national networking days for pharmacists and pharmaconomists have been held, where good initiatives are shared to all the regions and hospital pharmacies in Denmark.
What next?
The group is working on national standards for competency development of clinical pharmacy staff. Other logistics tasks will be scrutinized to see whether resources can be found for further investment in clinically related activities.
More benchmarking will be carried out, measuring other clinical and ward pharmacy activities throughout Denmark.
The work has just begun!



























