25% sodium thiosulphate in the topical treatment of calciphylaxis
Pdf

European Statement
Production and Compounding
Author(s)
VIRGINIA PUEBLA GARCIA, MARIA MOLINERO MUÑOZ, ANA ANDREA GARCIA SACRISTAN, JAVIER CORAZON VILLANUEVA, LIDIA YBAÑEZ GARCIA, NATALIA SANCHEZ-OCAÑA MARTIN, PALOMA PASTOR VARA, MARIA FERNANDEZ-VAZQUEZ CRESPO, JOSE MANUEL MARTINEZ SESMERO
Why was it done?
Calciphylaxis is a vascular disorder characterised by the accumulation of calcium in the small blood vessels of the skin and adipose tissue. There is an imbalance in calcium metabolism which causes calcium to be deposited in the arterioles favouring thrombosis in the residual lumen of these vessels. It presents with severe painful skin lesions which progress to ulcers. It mostly affects patients on renal replacement therapy.
What was done?
To describe the making process of a 25% sodium thiosulphate ointment (ST25%) requested by the Nephrology Department as an off-label use for the topical treatment of calciphylaxis in a patient who was unable to use intravenous sodium thiosulphate (ST) due to haemodynamic instability.
How was it done?
We initially performed an online literature search of databases related to raw materials and excipients, experience of use with formulas prepared by other hospitals as well as articles related to calciphylaxis.
For the production and quality control, the Standard Operating Procedure (SOP) for ointments described in the National Formulary was followed. To establish the risk level of the preparation and the expiry date, a risk matrix was used according to the Guide to Good Pharmacy Preparation Practice (GBPP).
What has been achieved?
It was decided to make a ST25% ointment. Composition for 100 g: ST 25 g (active ingredient), glycerine 10 g (humectant, cosolvent), pure lanolin 32.5 g and white filmy petrolatum 32.5 g (vehicles).
Production: the ST crystals were pulverised in a mortar. Glycerine was gradually added on top of the ST until a uniform whitish paste free of crystals was formed. At the same time, lanolin and filmy petrolatum was mixed in the final container with the help of an emulsifier. Finally, the paste formed with ST and glycerine was added to the lanolin-Vaseline mixture and stirred in the emulsifier until a homogeneous ointment was obtained.
A yellowish ointment with a homogeneous appearance, oily texture and no crystals was obtained.
Expiry date: 30 days after opening. Low-risk preparation.
What next?
Calciphylaxis could be treated after intolerance to intravenous sodium thiosulphate by developing an ointment. The pharmacist through magistral formulation can provide pharmaceutical alternatives in situations where the use of commercially available medicines is not possible.
Homemade personalized 3D printed guides for mandibular reconstruction – A pharmacist feedback
European Statement
Production and Compounding
Author(s)
Quentin Misandeau, Romain Bosc, Muriel Paul, Valérie Archer, Lionel Tortolano
Why was it done?
The plastic surgery department bought two 3D printers in order to design and create custom-made medical devices. The main objective was to decrease the delay between mandibular cancer diagnosis and the surgery. The delays of production in the medical device manufacturers may exceed 2 weeks. Those delays are considered as a lost of chance for the patients.
What was done?
We helped our surgeons to secured the production procedure and reduce the risks for patients. We create a management quality system for supply, production, sterilization and using of our homemade personalized 3D printed guides.
How was it done?
First, we created a task force of pharmacist and surgeons.
The main work was a risk analysis with the FMECA method for all the steps in the procedure (software, tools, actors and materials).
What has been achieved?
Some correctives actions were taken. The riskier points were the plastic materials toxicity risk, the sterilization procedure that has been validated and the production traceability. A biocompatibility evaluation was performed. A clinical evaluation has been initiated in the same period.
What next?
Since the new medical device regulatory (2017 /745 CE) was published in 2017, the article five, that regulate the 3D printing in hospital, changes the possibilities. In fact, as personalized 3D printed guides are available on the EU market, homemade personalized 3D printed guides for mandibular reconstruction could be not authorized anymore. The only way would be for the hospital to obtain the regulatory statut of manufacturer and comply with the essential requirements.
A new vancomycin formulation for oral use
European Statement
Production and Compounding
Author(s)
Mette Lethan, Tove Hansen, Trine Schnor, Louise Rasmussen Duckert
Why was it done?
Oral vancomycin 125 mg four times a day for 10 days, is the common treatment for antibiotic-associated clostridium difficile colitis. As solid oral formulations are unsuitable due to strong diarrhea, an i.v. formulation in a diluted form (10 mg/ml) is used. However, several issues with that use, required the need for creating a new formulation.
The product is used for treatment of kids and adults, often with nausea. The low strength requires large volumes of solution and with no flavoring the liquid is very bitter.
Furthermore, the current solution has a limited stability causing difficulties as it is often desired to treat the patients at home. Therefore, a wish arose for a new formulation with a higher concentration, better stability and improved organoleptic qualities.
What was done?
A new oral formulation of Vancomycin was developed to improve the treatment of antibiotic-associated clostridium difficile colitis. The new formulation consists of the active ingredient (API) Vancomycin as a powder with a solvency for dissolving prior to use.
How was it done?
To obtain the best stability it was selected to make a new formulation consisting of a premeasured API, with a solvency ready to mix before use, to obtain a final strength of 50 mg/ml and a volume equivalent to 48 hours of doses.
Vancomycin in pharmacopeia quality was acquired and analyzed. A solvency was formulated mostly consisting of water, conservation and orange flavoring. A test was conducted with a weighed-out API to ensure that it was dissolvable with the solvency in the selected packaging.
What has been achieved?
A product achieving the wanted changes was tested and made. Making a formulation consisting of a premeasured powder creates the possibility of a higher durability. When mixed with the flavored solvency, the wanted strength is achieved.
The new formulation can be stored at room temperature before dissolving. It can be dissolved by the patient before use and kept refrigerated until the full volume is used. The product is easy for the patient to handle and therefore enables treatment in the patients own home.
What next?
A new product was made. Final use by patients will be tested for ease of use and potential home treatment.
THE RISK MANAGEMENT OF THE PHARMACY PREPARATIONS IN THE HOSPITAL PHARMACIES (submitted in 2019)
Pdf

European Statement
Production and Compounding
Author(s)
ADRIANA DURCANSKA
Why was it done?
The quality and safety standards of pharmacy preparations are not harmonised throughout Europe. They fall under the national competencies of individual European countries.
What was done?
The quantitative risk assessment of the pharmacy preparations for stock in hospital pharmacies (HPs) in accordance with Resolution EDQM CM / Res (2016) 1; to specify the decision criteria for the risk assessment; the risk management of the pharmacy preparations for stock in the country; to design a check list of the risk assessment for extempore preparations.
How was it done?
Out of the total number of 53 hospital pharmacies contacted, 5 pharmacies sent a suitable file.
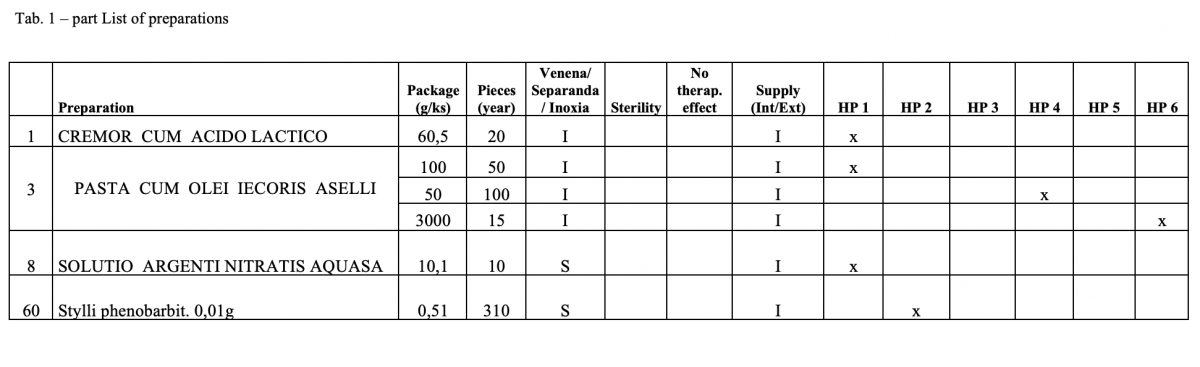
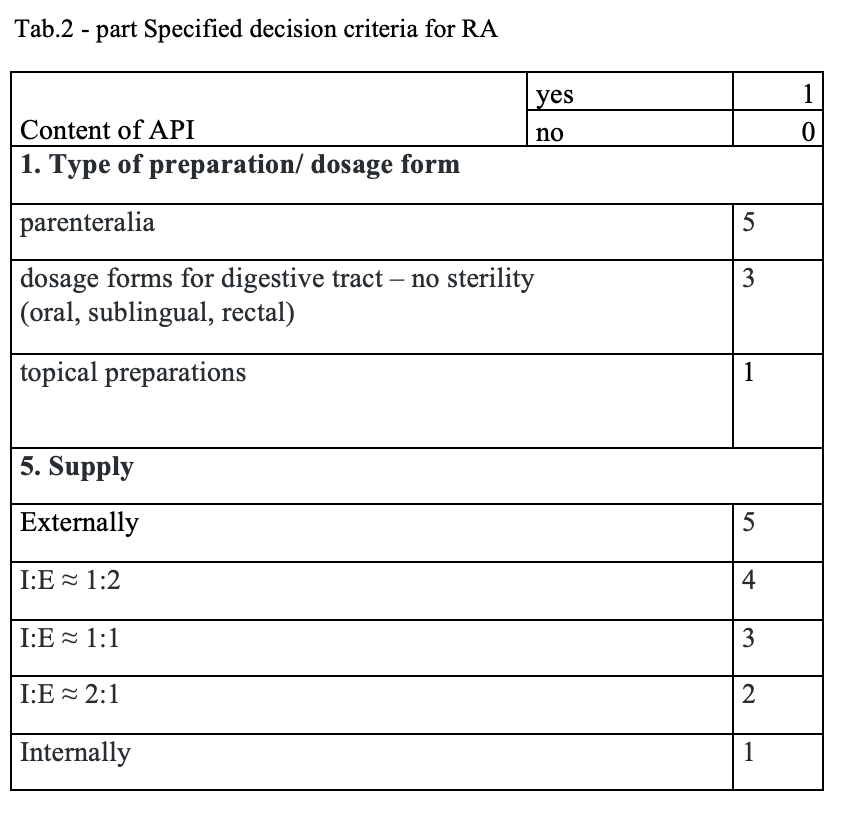
What has been achieved?
A total of 170 types of medicines are being prepared in HPs. One HP had the result of the risk ≥ 100 when preparing ophthalmic medicines. Annex A is a check list designed to assess the risk of extempore preparations.
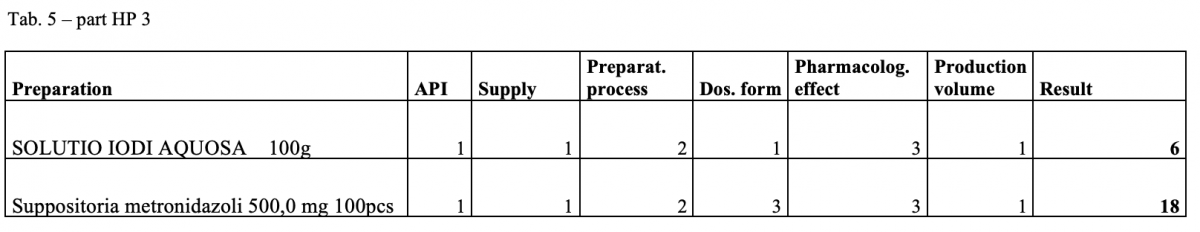
What next?
The management is and will be forced to consider its introduction or to use another model: hospital – GMP / outsourcing / central pharmacy preparing and distributing. The aim of using the document in hospital pharmacies of the country.
DEVELOPMENT OF NEW PRODUCTION WHEN NEITHER PACKAGING NOR SOME OF THE RAW MATERIALS CONFORM TO EUROPEAN STANDARDS
Pdf

European Statement
Production and Compounding
Author(s)
Katrine Bødker Rubach-Larsen, Anne Rungø, Anette Eskildsen, Lone Skovhauge
Why was it done?
A research team at the MR Centre (MRC2) wished to set up the production of Pharmacy Kits, but had no prior experience of, or licence to, manufacture drugs. Thus, the hospital pharmacy was asked to participate in the development of such production.
What was done?
A new MR-scanning technology, hyperpolarisation, for the quantification of metabolic processes with an extremely high sensitivity enables physicians early detection of treatment effects in, for example, cancer and diabetes. A so-called Pharmacy Kit is used in the hyperpolarisation process and consists of a specially designed packaging with tubes, vessels and filters containing the contrast agent and buffer solutions. The objective for the hospital pharmacy1 was to manufacture Pharmacy Kits complying with Good Manufacturing Practice (GMP), though neither packaging nor two of the raw materials conformed to European standards.
How was it done?
The MRC research team presented the hospital pharmacy with the desired combination of compounds and the packaging required for Pharmacy Kit production. The task for the hospital pharmacy was then to set up a manufacturing process that met these requirements and complied with the guidelines for GMP. A production complying with GMP was developed in close collaboration with the MRC and an ongoing contact with the Danish Medicines Agency. During the process the hospital pharmacy carried out its own microbiology test in order to determine if, and for how long, the non-CE-marked packaging could store the contrast agent and buffer solutions. Risk assessment of the raw materials not found in the European Pharmacopeia were conducted. The method investigated by the MRC already takes place at a few other places in and outside of Europe. Experiences from these production sites were implemented and expanded with process optimisation, and specially designed equipment for the production.
What has been achieved?
Due to a strong inter-professional collaboration between the MRC and the hospital pharmacy and due to qualified risk assessments, it was possible to set up a production of Pharmacy Kits according to GMP.
What next?
When researchers contact hospital pharmacies with new ideas, we have to be willing to work with GMP in a different way by applying knowhow and risk assessments in order to ensure developments within the healthcare system.
1. Hospital Pharmacy Central Region, Production, Aarhus, Denmark.
2. MR Centre, Aarhus University Hospital, 8200 Aarhus N, Denmark



























