The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
Students sign up for the EAHP-EPSA Student Science Award!
 The European Association of Hospital Pharmacists (EAHP) is proud to announce the opening of the next round of the EAHP-EPSA Student Science Award. This prestigious prize recognises and honours the best scientific research authored by a pharmacy student or recent graduate. Since 2011 the EAHP-EPSA Student Science Award is offered to one member of the European Pharmaceutical Students’ Association (EPSA) who has conducted research in the field of hospital and/or clinical pharmacy.
The European Association of Hospital Pharmacists (EAHP) is proud to announce the opening of the next round of the EAHP-EPSA Student Science Award. This prestigious prize recognises and honours the best scientific research authored by a pharmacy student or recent graduate. Since 2011 the EAHP-EPSA Student Science Award is offered to one member of the European Pharmaceutical Students’ Association (EPSA) who has conducted research in the field of hospital and/or clinical pharmacy.
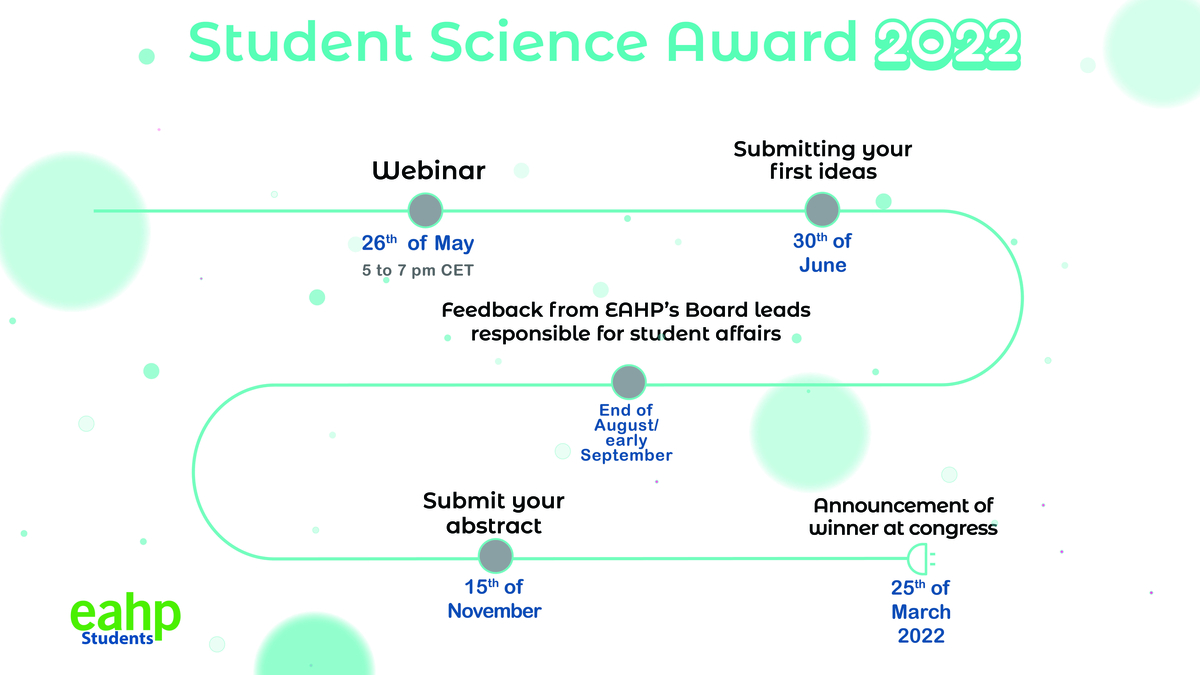
To enter into the competition, students and recent graduates are required to attend the webinar “How to write an abstract”, hosted by EAHP’s Directors of Professional Development Ana Lozano, Claudia Plesan and Piera Polidori. The webinar will take place on the 26th of May 2021 from 17.00 to 19.00 CET. Students and recent graduates can register by sending an email with their first and last name to intern@eahp.eu
After the webinar, participating students and recent graduates can obtain detailed feedback on their work prior to submitting their final abstract by 15th November. The abstracts are reviewed in accordance with the criteria of innovation, originality, and contribution to the development of hospital pharmacy. The author of the winning abstract will receive a complimentary registration to the 26th Congress of EAHP, including the coverage of travel and accommodation expenses (up to 500 Euros), as well as official recognition at the EAHP Congress closing ceremony on the 25th of March 2022.
More information about the EAHP-EPSA Student Science Award HERE
Public consultation for European Health Data Space is open
 The European Commission has launched a public consultation on the European Health Data Space (EHDS). Stakeholders are encouraged to share their comments until the 26th of July.
The European Commission has launched a public consultation on the European Health Data Space (EHDS). Stakeholders are encouraged to share their comments until the 26th of July.
The purpose of the EHDS is to promote health-data exchange and support research on new preventive strategies, as well as on treatments, medicines, medical devices and outcomes. The public consultation focuses on the access to and use of health data for healthcare provision, research and innovation, policy-making and regulatory decision and on fostering a genuine single market for digital health services and products, including innovative ones. By participating, you can provide important insights, opinions and evidence to support the impact assessment accompanying the EHDS proposal on the problems to be tackled, the policy options to be considered and their likely impacts.
Access the consultation HERE
Commission proposes EU Strategy for the development and availability of therapeutics
 Last week, the European Commission released its strategy on COVID-19 therapeutics which aims at supporting the development and availability of much-needed treatment options, including those for ‘long COVID’. The strategy covers the full lifecycle of medicines ranging from research, development over manufacturing to procurement and deployment.
Last week, the European Commission released its strategy on COVID-19 therapeutics which aims at supporting the development and availability of much-needed treatment options, including those for ‘long COVID’. The strategy covers the full lifecycle of medicines ranging from research, development over manufacturing to procurement and deployment.
The Strategy proposes clear actions and targets, including authorising three new therapeutics to treat COVID-19 by October 2021 and possibly two more by end of the year. By next month, the European Commission will draw up a portfolio of 10 potential COVID-19 therapeutics to identify the five most promising ones. It will organise matchmaking events for industrial actors involved in therapeutics to ensure enough production capacity and swift manufacturing. It is envisioned that new authorisations, rolling reviews and joint procurement contracts will be up and running before the end of the year.
Read the Strategy on COVID-19 Therapeutics HERE
EJHP: Pharmaceutical interventions in the emergency department: cost-effectiveness and cost-benefit analysis

The original research published in the online edition of the European Journal of Hospital Pharmacy (EJHP) analyses pharmaceutical interventions (PI) in emergency departments, to assess their clinical relevance, cost-effectiveness and potential economic benefits. The authors designed a 5-month observational prospective study of PI in the emergency department (ED) of a 330-bed hospital in Spain and used statistical analysis to study the relationship between the relevance of PI and age, gender, the number of interventions per patient, and whether or not the drug was on the High-Alert Medications ISMP list. The results show that clinical pharmacist can positively identify and reduce medication errors and costs associated, considering the number of interventions observed and those of clinical relevance.
Read the article HERE
 [COVID-19 Updates]
[COVID-19 Updates]
EAHP’s COVID-19 Resource Centre
To assist its member associations and individual hospital pharmacists in this critical time with the provision of the best possible care for patients, EAHP has decided to gather and make available information on COVID-19 relevant for the hospital pharmacy profession.
Access the Resource Centre HERE
American Association for the Study of Liver Diseases (AASLD) – Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement
AASLD has put together a template for developing clinical recommendations and policies to mitigate the impact of the COVID-19 pandemic on liver patients and health care providers, and for providing safe and optimal care in response to changes in our work and surrounding environment.]
Access the document HERE
British Society for Haematology – Guidance from the Expert Haematology Panel (EHP) on Covid-19 Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT)
The guideline aims at providing support for the management of COVID-19 Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT), based on the experience of managing the initial cases, alternative similar conditions and the theoretical risks and benefits of intervention.
Access the guidance HERE
NICE – Clinical guide for the management of palliative care in hospital during the coronavirus pandemic
NICE provides a guideline to support best practice palliative care for all patients who require this, either with pre-existing palliative care needs or because of coronavirus infection.
Access the guideline HERE
The International Journal of Clinical Practice – Evaluation of the role and usefulness of clinical pharmacists at the Fangcang Hospital during COVID-19 outbreak
This study was a retrospective study to evaluate the usefulness of clinical prevention and control measures of clinical pharmacists at Jianghan Fangcang Hospital.
Read the article HERE
The European Statements of Hospital Pharmacy in videos!

EAHP has developed a series of videos for the different sections of the European Statements of Hospital Pharmacy to help you better understand how hospital pharmacists should be involved in the delivery of hospital pharmacy services. Watch them here!
___________________________________________________________________________________

Consultations
European Commission – Public Consultation: European Health Emergency Preparedness and Response Authority (HERA)
The COVID-19 pandemic demonstrated the need for coordinated EU level action to respond to health emergencies. It revealed gaps in foresight, including demand/supply dimensions, preparedness and response tools. A European HERA is a central element for strengthening the European Health Union with better EU preparedness and response to serious cross-border health threats, by enabling rapid availability, access and distribution of needed countermeasures. Through the public consultation you can express your views on HERA before the Commission finalises its proposal.
Deadline – 12th May 2021
Access the consultation HERE























 The European Association of Hospital Pharmacists (EAHP) is proud to announce the opening of the next round of the EAHP-EPSA Student Science Award. This prestigious prize recognises and honours the best scientific research authored by a pharmacy student or recent graduate. Since 2011 the EAHP-EPSA Student Science Award is offered to one member of the European Pharmaceutical Students’ Association (EPSA) who has conducted research in the field of hospital and/or clinical pharmacy.
The European Association of Hospital Pharmacists (EAHP) is proud to announce the opening of the next round of the EAHP-EPSA Student Science Award. This prestigious prize recognises and honours the best scientific research authored by a pharmacy student or recent graduate. Since 2011 the EAHP-EPSA Student Science Award is offered to one member of the European Pharmaceutical Students’ Association (EPSA) who has conducted research in the field of hospital and/or clinical pharmacy.  The European Commission has launched a public consultation on the European Health Data Space (EHDS). Stakeholders are encouraged to share their comments until the 26th of July.
The European Commission has launched a public consultation on the European Health Data Space (EHDS). Stakeholders are encouraged to share their comments until the 26th of July.  Last week, the European Commission released its strategy on COVID-19 therapeutics which aims at supporting the development and availability of much-needed treatment options, including those for ‘long COVID’. The strategy covers the full lifecycle of medicines ranging from research, development over manufacturing to procurement and deployment.
Last week, the European Commission released its strategy on COVID-19 therapeutics which aims at supporting the development and availability of much-needed treatment options, including those for ‘long COVID’. The strategy covers the full lifecycle of medicines ranging from research, development over manufacturing to procurement and deployment.
 [COVID-19 Updates]
[COVID-19 Updates]

