The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
You can subscribe to receive the EAHP EU Monitor by email HERE.
1st EAHP Synergy Certification Course

The European Association of Hospital Pharmacists (EAHP) is launching its first Synergy Certification Course. The event will take place in Belgrade, Serbia on 23rd and 24th February 2019. It is organised by our colleagues from the Serbian Hospital Pharmacy Section and will be held together with the “3rd Symposium on Hospital Pharmacy Practice in Serbia”.
To ensure that many hospital pharmacists from neighbouring countries, such as Bulgaria, Bosnia and Herzegovina, Croatia, the Former the former Yugoslav Republic of Macedonia (FYROM), Greece, Montenegro, Romania and Slovenia, can join the Synergy Certification Course, the event will be hosted in both English and Serbian. The topic of this brand new course is “Information Technology Systems support in antibiotic prescription and antibiotic stewardship”. On Saturday, best practice examples will be shared by speakers from Austria, Belgium, Croatia and Greece. The afternoon is dedicated to parallel workshops that will be run in World Café Style with the aim to strengthen the newly acquired information. On Sunday, information from EAHP’s 2018 Academy Seminar programme will be disseminated. Participants will be provided with the opportunity to learn more about therapeutic drug monitoring which can be used as a tool for therapy optimisation. In addition, in the field of medicines shortages, causation and approaches to improvement will be shared.
Interested hospital pharmacists are encouraged to sign up for this exciting event!
See programme HERE
Register HERE
Accomodation form HERE
ePrescription
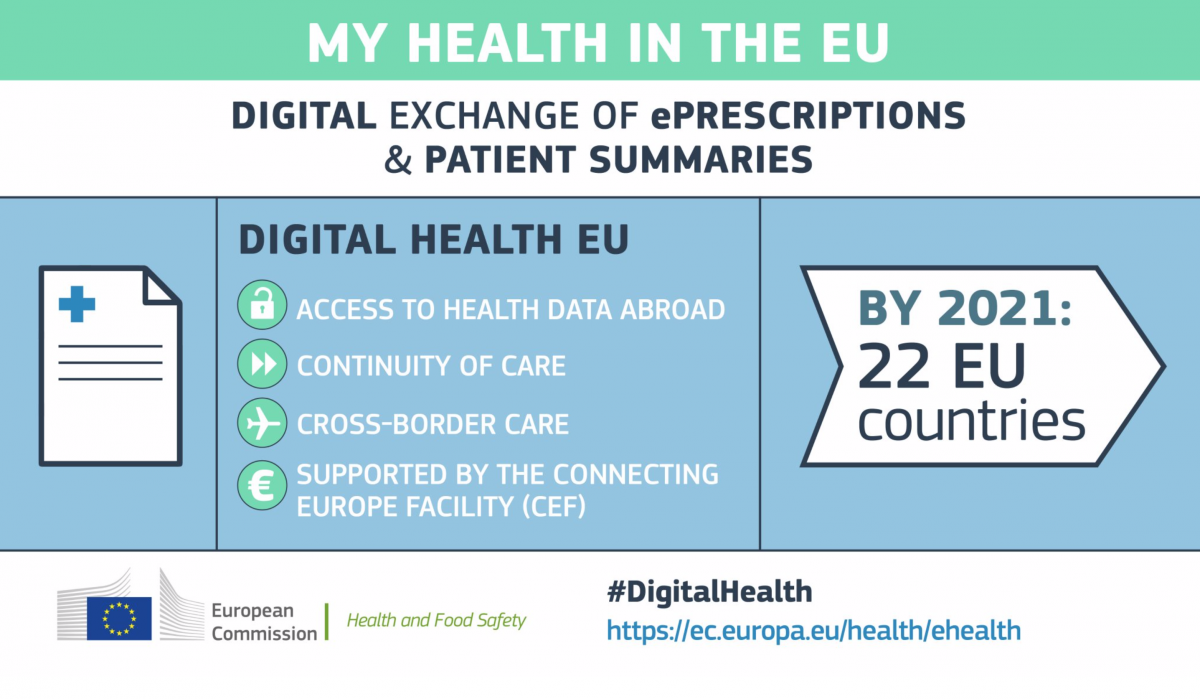
Since 21stJanuary, patients in Finland and Estonia are able to use digital prescriptions issued by their physician when visiting a pharmacy. Finland and Estonia have signed an agreement that enables this exchange. In line with the objectives of European Commission’s policy on Digital Health and Care, the ePrescriptions exchanged between Estonia and Finland are visible electronically to participating pharmacists in the receiving country via the new eHealth Digital Service Infrastructure. Due to this novelty, patients will no longer have to provide a written prescription.
The implementation of this ePrescription initiative has been made possible by Directive 2011/24which ensures continuity of care for European citizens across borders. The exchange of patient summaries of foreign citizens, which is currently tested by Czechia and Luxembourg, as well as eDispensation fall also under the scope of this Directive. By the end of 2021, 22 Member States will be part of the eHealth Digital Service Infrastructure and are expected to exchange ePrescriptions and patient summaries. In 10 Member States, namely Finland, Estonia, Czechia, Luxembourg, Portugal, Croatia, Malta, Cyprus, Greece and Belgium, these exchanges will already be facilitated by the end of 2019.
Learn more about the initiative HERE
Access the Q&A document HERE
Romanian Council Presidency programme

In mid-January, the Romanian Council Presidency released its programme for the coming 5,5 months. As outlined in the EU Monitor of 10thJanuary 2019, Romania’s Presidency will overall focus on four main priorities: Europe of convergence, a safer Europe, Europe as a strong global actor and Europe of common values.
In relation to public health, the Presidency Programme underlines the need to guarantee access to healthcare for all European citizens, to fight antimicrobial resistance, to enhance vaccination coverage, to reduce medication abuse and to improve the control of transmissible diseases. Patients are at the centre of the actions in the field of health. Consequently, the Romanian Presidency plans to organise debates focusing on patient mobility which encourage cooperation between the different EU Member States, especially in the area of rare diseases.
Patient’s access to medicines is one of the cornerstones of Romania’s presidency programme. The EAHP welcomes this initiative, in particular since the problem of medicines shortages has become more troublesome in the past 4 years, as outlined in EAHP’s 2018 Medicines Shortages Survey Report. Healthcare digitisation and the continuation of the negotiations on the proposal for a Regulation on the coordination of health technology assessment will also be addressed during the upcoming months. Given the growing threat of antimicrobial resistance (AMR), the Romanian Presidency intends to put forward Council conclusions on the fight against AMR and hospital-acquired infections.
See programme HERE
ECDC survey announcement
 On 28thJanuary, the European Centre for Disease Prevention and Control (ECDC) will be cascading the link to an ECDC-funded survey to assess healthcare workers’ antibiotic use and resistance. The aim is to have a robust return of 10,000+ responses across the EU/EEA member states with representation from healthcare workers (doctors, nurses and midwives, dentists, (hospital) pharmacists, other healthcare workers (e.g. hospital managers, allied health professionals, technicians)). ECDC is also seeking responses from health students.
On 28thJanuary, the European Centre for Disease Prevention and Control (ECDC) will be cascading the link to an ECDC-funded survey to assess healthcare workers’ antibiotic use and resistance. The aim is to have a robust return of 10,000+ responses across the EU/EEA member states with representation from healthcare workers (doctors, nurses and midwives, dentists, (hospital) pharmacists, other healthcare workers (e.g. hospital managers, allied health professionals, technicians)). ECDC is also seeking responses from health students.
The overall objectives of the study for ECDC are:
- To fill in gaps in terms of evaluation of communication campaigns targeting healthcare workers, and
- To gain a better understanding of their knowledge and perceptions to provide a base to support future needs in terms of policy and education changes.
The survey will be open between 28thJanuary and 14thFebruary 2019. Please note that the survey will be made available in different European languages.
Access ECDC’s website HERE
EURORDIS – 3rd Multi-Stakeholder Symposium on Improving Patients’ Access to Rare Disease Therapies

The EAHP has partnered with EURORDIS – the voice of rare disease patients in Europe – for its 3rd Multi-Stakeholder Symposium on Improving Patients’ Access to Rare Disease Therapies. The event will take place in Brussels on 13th and 14th February under the theme “Let’s make a pact to ensure patients’ sustainable access to rare disease therapies”.
As outlined in the last issue of the EU Monitor, the event will be accompanied by two pre-Symposium webinars which will both take place in January. On 24th January, from 15.00 and 16.00, interested parties are invited to join a webinar focusing on the aspects of value assessment of therapies and current challenges for accurate appraisal. The second webinar, covering research and development for rare diseases therapieswill be held on 31stJanuary from 15.00 to 16.00.
More information about the 3rd Multi-Stakeholder Symposium HERE
EJHP: Analysis of the impact of antimicrobial management and rational use of antibiotics

The online first edition of the European Journal of Hospital Pharmacy (EJHP) recently published an original article addressing the impact of antimicrobial management and rational use of antibiotics. It was concluded after the analysis that scientific management can promote the rational use of antibiotics, reduce the expense of drug use and slow the development of drug resistance. The authors also underlined that further optimisation of the prescription of antibiotics is needed to improve the level of drug treatment.
Read more HERE
[EAHP Statement Corner]
Join us at the 24thCongress of the EAHP and learn more about the Statements!

Visit the Statement Implementation team at EAHP’s 24thCongress in Barcelona, Spain to learn more about the 44 European Statements of Hospital Pharmacy, EAHP’s Statement Implementation Learning Collaborative programme (SILCC) and the Self-assessment Tool (SAT). Our team will be waiting for you at the EAHP Booth in the exhibition area to answer all your questions related to the Statements. Make sure to sign up for our Congress by 31stJanuary to benefit from our discounted registration rate.Register HERE for EAHP’s 24thCongress
————————————————————————————————

Consultations
EDQM – European Paediatric Formulary consultation
The European Directorate for the Quality of Medicines & HealthCare (EDQM) has launched a public consultation on the first two pilot monographs and on two general texts for its European Paediatric Formulary. European pharmacists and paediatricians are invited to share their feedback on the draft monographs on Hydrochlorothiazide oral solution and on Sotalol oral solution.
Deadline – 31stJanuary 2019
More information HERE
EMA – Draft guideline on clinical investigation of medicinal products in the treatment of epileptic disorders
The present document is a third revision of the existing guideline. The main changes to the existing guideline include incorporation of the new classification / definitions of seizure types and epilepsies, the acceptance of add-on studies in support of a monotherapy claim on a case-by-case basis, the inclusion of new sections on neonates and status epilepticus and other changes related to paediatric developments. The scope of this document is restricted to treatment of seizures in epileptic disorder although there are some remarks concerning non-seizure features of epilepsy syndromes.
Deadline – 17th February 2019
More information HERE
Survey on patients opinion about drug shortages
COST Action CA 15105 on medicines shortages invites you to participate in a qualitative study regarding patients’ perspective about medicine shortages during hospital stay. Hospital pharmacists are encouraged to question interested patients who are willing to share their experience with medicines shortages. Questions in relation to this survey activity can be address by email to Darija Kuruc Poje (darijakuruc21@gmail.com).
Deadline – 1stMarch 2019
Access the survey HERE
EMA – Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections
The EMA has launched a consultation on the revision of its guideline on the evaluation of human medicines indicated for the treatment of bacterial infections. Antimicrobial resistance is a global public health problem. Regulators in the European Union, the United States and Japan have had extensive discussions over the last few years to explore and agree how to align as much as possible their respective data requirements so that medicine developers can design clinical trials that meet the evidence needs of multiple regulatory agencies. The revised guidance reflects the outcome of these discussions.
Deadline – 31stJuly 2019
Access consultation HERE




























 On 28thJanuary, the European Centre for Disease Prevention and Control (ECDC) will be cascading the link to an ECDC-funded survey to assess healthcare workers’ antibiotic use and resistance. The aim is to have a robust return of 10,000+ responses across the EU/EEA member states with representation from healthcare workers (doctors, nurses and midwives, dentists, (hospital) pharmacists, other healthcare workers (e.g. hospital managers, allied health professionals, technicians)). ECDC is also seeking responses from health students.
On 28thJanuary, the European Centre for Disease Prevention and Control (ECDC) will be cascading the link to an ECDC-funded survey to assess healthcare workers’ antibiotic use and resistance. The aim is to have a robust return of 10,000+ responses across the EU/EEA member states with representation from healthcare workers (doctors, nurses and midwives, dentists, (hospital) pharmacists, other healthcare workers (e.g. hospital managers, allied health professionals, technicians)). ECDC is also seeking responses from health students.



