The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
You can subscribe to receive the EAHP EU Monitor by email HERE.
3rdmeeting of EAHP’s Implementation Ambassadors

From 15 to 17 November, the European Association of Hospital Pharmacists (EAHP) held the 3rd EAHP meeting and training of Implementation Ambassadors. 24 ambassadors representing 21 national member associations participated in the event.
Implementation Ambassadors are highly motivated and enthusiastic hospital pharmacists that are appointed by their national member association to help their country move towards the implementation of the European Statements of Hospital Pharmacy. Their main role can be summed up as follows:
- Act as primary link in between EAHP, their national associations and the implementation activities done within their countries.
- Convey to EAHP the needs and priorities of their countries regarding Statement implementation.
- Build resources that will help hospital pharmacists, healthcare professionals and other relevant stakeholders to implement the Statements within their hospitals.
The 3rd meeting of our Implementation Ambassadors was a perfect opportunity to brainstorm and discuss about the next steps of the project, as well as a perfect venue for the group to convey to EAHP their national needs and ideas. The training following the meeting was facilitated by KayHill consultancy. It focused on communication strategies. In particular, Implementation Ambassadors learned about the best ways to approach stakeholders in order to promote the benefit of implementing the Statements for the patient.
EAHP wants to take this opportunity to thank all Implementation Ambassadors for their great work and active participation. Stay tuned for more news on the implementation project!
Learn more about the Implementation Ambassadors HERE
More information about the Statements HERE
EAHP’s Scientific Committee Call

The EAHP has launched a call for the expression of interest addressed to all hospital pharmacists and scientists who wish to be considered for membership in EAHP’s Scientific Committee. This Call for Expressions of Interest closes on 5 December 2018.
More information on the responsibilities of the Scientific Committee, the selection criteria and the application process are available HERE
New rules in the veterinary sector to help battle AMR

On 26 November the European Council gave the green light for new rules on veterinary medicines and medicated feed. These new rules aim at improving the fight against antimicrobial resistance (AMR) in the veterinary sector.
The new Regulation on veterinary medicinal products repeals Directive 2001/82/EC. In line with the European Commission’s One Health Approach on AMR, it will promote a prudent and responsible use of antimicrobials in the veterinary field. Moreover, the new Regulation simplifies the marketing authorisation procedure for veterinary medical products in order to reduce the administrative burden for companies, especially small ones, strengthens pharmacovigilance and controls as well as improves the protection of the European consumers against the risk of the spreading of AMR through imports of animals and products of animal origin. The rules will become applicable in the next three years.
The new Regulation on medicated feed that will repeal the outdated Directive 90/167/EEC aims at
- bringing into action a ban on the preventive use of antimicrobials via medicated feed;
- restricting the veterinary prescriptions with antimicrobials; and
- establishing harmonised limits for antimicrobials in ordinary feed.
In addition, it will set EU harmonised standards for manufacturing safe medicated feed and create a legal framework for safe manufacturing and distribution of medicated feed for pets.
More information HERE
The state of European health
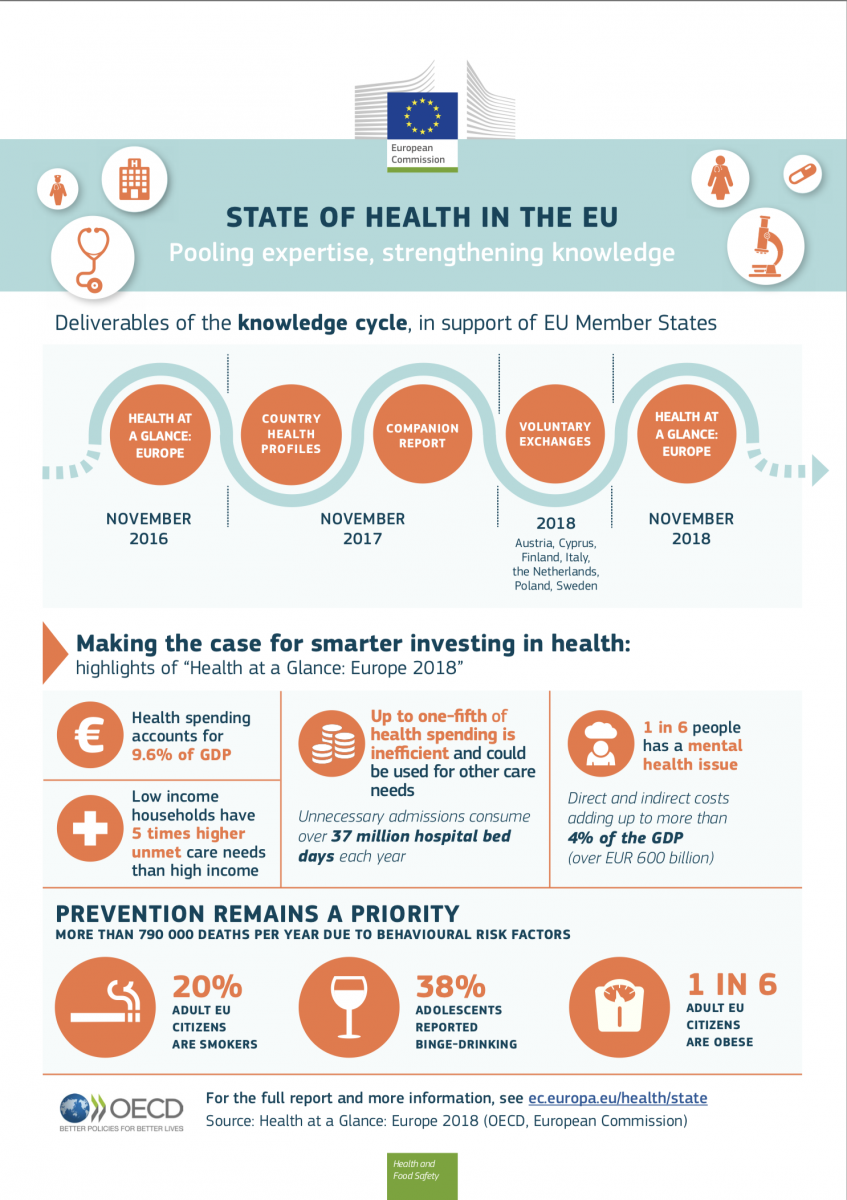
On 22 November, the European Commission and the Organisation for Economic Cooperation and Development (OECD) issues their joint report ‘2018 Health at a Glance: Europe 2018 – State of Health in the EU cycle’. The results which are based on information provided by the EU28, candidate countries and theEuropean Free Trade Association (EFTA) countries provide an analysis of the state of health of EU citizens and the performance of EU health systems.
Overall, the report outlines that gains in life expectancy have slowed down and that large disparities continue to persist. In relation to health systems, it was underlined that 20 % of health spending could be reallocated for better use. Optimisation are needed to reduce wasteful spending and to make health systems more effective and resilient. For the hospital sector it was highlighted that many admissions could be avoided with better management of chronic conditions in the community. The report also puts a stronger emphasis on prevention and calls for improvements in the mental health sector. Preventive actions should for instance tackle risk factors like smoking, alcohol and obesity as well as help with the reduction of premature mortality.
The publication of the report kicked-off the new state of health cycle. The second phase will commence in 2019 when the country health profiles of all EU countries are released by the OECD and the European Observatory on Health Systems and Policies. They will be presented alongside a companion report that offers conclusions. The current cycle will be concluded by a voluntary exchange with members states.
Read the report HERE
More information HERE
EDQM – European Paediatric Formulary consultation

The European Directorate for the Quality of Medicines & HealthCare (EDQM) has launched a public consultation on the first two pilot monographs and two general texts for its European Paediatric Formulary, a pan-European collection of formulations for extemporaneous preparations that are either currently described in national formularies or are already well-established in European countries. The Formulary will be made available free of charge and is intended as an information-sharing platform that will help fill the gap until corresponding medicines that have been approved for use in the paediatric population are available.
The first texts were published for public consultation in October. All stakeholders are invited to provide their comments on two texts introducing the formulary and its aims and on the two pilot monographs, Hydrochlorothiazide 0.5 mg/mL oral solution and Sotalol hydrochloride 20 mg/mL oral solution. The closing date for comments is the end of January 2019.
European Paediatric Formulary monographs reproduce information from national and other existing well-established monographs but may also contain data from other monographs or literature references. For example, the monograph on Hydrochlorothiazide oral solution is based on the Dutch FNA while the monograph on Sotalol hydrochloride oral solution is drawn from the German NRF.
The monographs share a common format, opening with a definition section followed by the qualitative and quantitative composition of the formulation, additional information (which includes any excipients or electrolytes of potential concern and information on osmolality, compatibility or bioavailability) and a detailed production section describing the compounding steps and in-process control tests to be carried out to ensure the reproducibility of the final formulation. A quality control section then provides comprehensive information about all the tests available for the preparation described, although it is understood that the pharmacist will determine which tests to perform depending on the scale of production and on national and regional legislation. Each monograph ends with information on storage and with references highlighting the main source.
The intention is to continue building the online formulary by adding other available preparations that fulfil the criteria for inclusion. The dedicated Working Party, which comprises 17 experts from hospital pharmacies, academia and national authorities in 14 countries, has already started work on oral liquids of Furosemide, Azathioprine, Isoniazide, Ranitidine, Omeprazole and Chloral hydrate. An oral vehicle and Oxybutynin intravesical solution are also in the pipeline.
As part of the ongoing public enquiry, pharmacists and paediatricians are invited to comment on the content of the monographs and on any possible alternative formulations they prepare before 31stJanuary 2019.
More information and access to the consultation HERE
EJHP: Incidence and prevalence of intravenous medication errors in the UK: a systematic review
 The online first edition of the European Journal of Hospital Pharmacy (EJHP) recently published a systematic review focusing on incidence and prevalence of intravenous medication errors in the UK. The study aims at estimating the number of intravenous medication errors in the UK National Health Service. It was concluded that intravenous medication errors are common in the UK, with half these of errors related to medication administration. National strategies have been put in place to address this issue. However, more needs to be done to reduce administration errors, particularly wrong rate errors.
The online first edition of the European Journal of Hospital Pharmacy (EJHP) recently published a systematic review focusing on incidence and prevalence of intravenous medication errors in the UK. The study aims at estimating the number of intravenous medication errors in the UK National Health Service. It was concluded that intravenous medication errors are common in the UK, with half these of errors related to medication administration. National strategies have been put in place to address this issue. However, more needs to be done to reduce administration errors, particularly wrong rate errors.
Read more HERE
[EAHP Statement Corner]
Become a SILCC fellow!

Do you want to visit hospitals from other member countries while learning about procedures linked to the European Statements? Then don’t hesitate to apply for the Statement Implementation Learning Collaborative (SILCC) programme!
Launched during the 23rd EAHP Congress in March 2018, the SILCC initiative allows hospitals pharmacists to visit hospitals from other EAHP member countries to receive training in procedures linked to the European Statements. Hospitals in Germany, Hungary, Italy Spain, Sweden and the United Kingdom are looking forward to hosting interested SILCC fellows. Visit our website to check more details about the training provided in these hospitals and to learn more about the application process.
————————————————————————————————

Consultations
COST Action CA 15105 – Disruptions, Vulnerabilities and Resilience Strategies in the Pharmaceutical Supply Chain
A researcher of the COST Action CA 15105 on medicines shortages invites you to participate in a consultation on disruptions, vulnerabilities and resilience strategies in the pharmaceutical supply chain. This study seeks to examine why the pharmaceutical supply chain in the UK is susceptible to the impact of disruptions and how resilience strategies can be used to mitigate the impact of these disruptions.
Deadline – 31st December 2018
More information HERE
Commission – Public consultation on the evaluation of the legislation on medicines for children and rare diseases
Healthcare professionals as well as private citizens are encouraged to share their experiences with and perspectives on access to orphan medicines in general, and on the role the EU Orphan Regulation plays in the development of orphan medicines.
Deadline – 4th January 2019
More information HERE
More information HERE
EDQM – European Paediatric Formulary consultation
The European Directorate for the Quality of Medicines & HealthCare (EDQM) has launched a public consultation on the first two pilot monographs and on two general texts for its European Paediatric Formulary. European pharmacists and paediatricians are invited to share their feedback on the draft monographs on Hydrochlorothiazide oral solution and on Sotalol oral solution.
Deadline – 31stJanuary 2019
More information HERE
EMA – Draft guideline on clinical investigation of medicinal products in the treatment of epileptic disorders
The present document is a third revision of the existing guideline. The main changes to the existing guideline include incorporation of the new classification / definitions of seizure types and epilepsies, the acceptance of add-on studies in support of a monotherapy claim on a case-by-case basis, the inclusion of new sections on neonates and status epilepticus and other changes related to paediatric developments. The scope of this document is restricted to treatment of seizures in epileptic disorder although there are some remarks concerning non-seizure features of epilepsy syndromes.
Deadline – 17th February 2019
More information HERE
Survey on patients opinion about drug shortages
COST Action CA 15105 on medicines shortages invites you to participate in a qualitative study regarding patients’ perspective about medicine shortages during hospital stay. Hospital pharmacists are encouraged to question interested patients who are willing to share their experience with medicines shortages. Questions in relation to this survey activity can be address by email to Darija Kuruc Poje (darijakuruc21@gmail.com).
Deadline – 1stMarch 2019
Access the survey HERE





























 The online first edition of the European Journal of Hospital Pharmacy (EJHP) recently published a systematic review focusing on incidence and prevalence of intravenous medication errors in the UK. The study aims at estimating the number of intravenous medication errors in the UK National Health Service. It was concluded that intravenous medication errors are common in the UK, with half these of errors related to medication administration. National strategies have been put in place to address this issue. However, more needs to be done to reduce administration errors, particularly wrong rate errors.
The online first edition of the European Journal of Hospital Pharmacy (EJHP) recently published a systematic review focusing on incidence and prevalence of intravenous medication errors in the UK. The study aims at estimating the number of intravenous medication errors in the UK National Health Service. It was concluded that intravenous medication errors are common in the UK, with half these of errors related to medication administration. National strategies have been put in place to address this issue. However, more needs to be done to reduce administration errors, particularly wrong rate errors.


