 The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
You can subscribe to receive the EAHP EU Monitor by email here.
EAHP welcomes Montenegro and FNSIP-BM as its newest members

The European Association of Hospital Pharmacists (EAHP) is delighted to announce further expansion of its membership, with the addition of a new country Montenegro, and the supplement of EAHP’s French membership platform to include the Fédération Nationale des Syndicats d’internes en Pharmacie et en Biologie Médicale (FNSIP-BM).
More information here.

EDQM publishes two new resolutions on pharmacy production and reconstitution
The European Directorate for the Quality of Medicines & HealthCare (EDQM) has published two new resolutions related to production and reconstitution of medicines in pharmacies.
EDQM is a Directorate of the Council of Europe (not to be confused with the European Union) that contributes to the protection of public health by elaborating harmonised standards to ensure the quality of medicines.
New EDQM Resolution CM/Res(2016)2 relates to good reconstitution practices in health care establishments for medicinal products for parenteral use.
The resolution recommends that all countries (currently 37) that are signatories to the Convention on the Elaboration of a European Pharmacopoeia should implement measures to guarantee that best practices are in place in health care establishments for the reconstitution of a medicinal product into a ready-to-use or ready-to-administer dosage form.
New EDQM Resolution CM/Res(2016)1 focuses on the quality and safety assurance requirements for medicinal products prepared in pharmacies for the special needs of patients. It represents an amendment to the pre-existing Resolution CM/ResAP(2011)1 to ensure consistency with the provisions of new Resolution CM/Res(2016)2 relating to medication reconstitution (see above).
The ultimate goals of the two new Resolutions are to promote public health through the harmonisation of requirements for medicinal preparations, support health professionals with appropriate guidance to prevent risk of health damage caused by drug preparation errors, and ensure that patient needs are fully met.
The full text of both resolutions are available here.
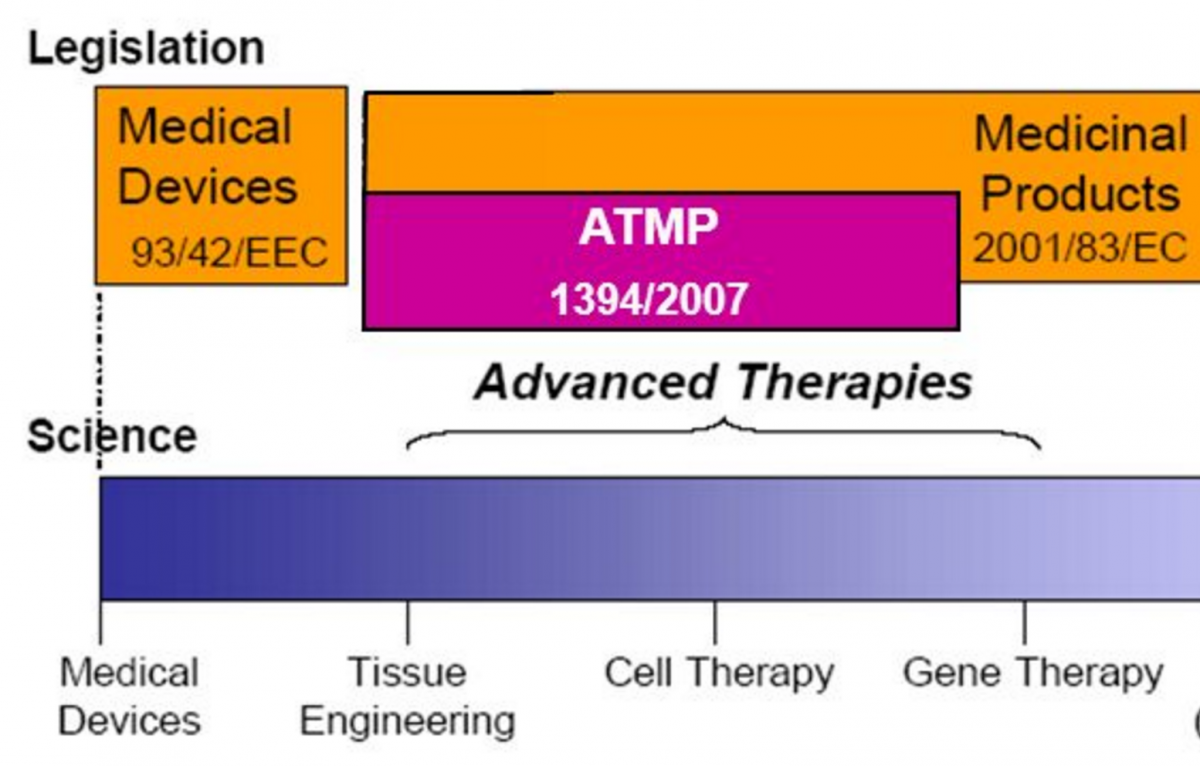 EMA and stakeholders explore how to improve patient access to ATMPs
EMA and stakeholders explore how to improve patient access to ATMPs
The European Medicines Agency (EMA) has published a report with conclusions on how the regulatory landscape for access to Advanced Therapy Medicinal Products (ATMPs) could be improved, including in respect to hospital manufacture dimensions.
The report summarises the deliberations of stakeholders at a one-day meeting in May 2016. Anne Black, a hospital pharmacist based at Newcastle upon Tyne Hospitals NHS Foundation Trust (UK), with specialist background in ATMPs, represented the European Association of Hospital Pharmacists.
A specific EU regulation for ATMPs has existed since 2007 and was intended to facilitate their development and introduction to market, via centralised authorisation procedures overseen by the European Medicines Agency. However, it has been noted that the number of ATMPs coming through the regulation’s procedures has been low. There have been seven EU-wide marketing authorisations provided for ATMPs since 2007, two of which have been subsequently withdrawn or suspended.
A major proposal at the meeting was for licensing requirements to take account of the particularities of ATMP manufacture. For example, participants noted that ATMP manufacture can take place at various sites and that, for some products, the later stages of production might need to take place close to the bedside. The implication is that all sites involved – hospitals included –would need to hold a manufacturing licence. A lack of harmonisation at national level was also noted, as some Member States allow hospitals to hold a manufacturing licence while others do not. Stakeholders therefore requested more information and harmonisation.
The meeting took place in the context of a European Commission consultation on new guidelines for ATMP Good Manufacturing Practice (GMP). Stakeholders considered that GMP requirements should be applied more flexibly for ATMPs at early development stages, as well as enable innovative manufacturing technologies such as bedside manufacturing and decentralised manufacturing.
Stakeholders also called for greater efforts towards international harmonisation of regulation, using the International Council for Harmonisation (ICH) as a means of achieving this.
A section of the report deals with the application of Article 28 (2) of the EU ATMP Regulation, the so called ‘hospital exemption’ clause. This permits Member States to use ATMPs in their territories without the need for marketing authorisation. This clause applies only to custom-made ATMPs used in a hospital setting for a specific patient. Such products are produced under the responsibility of a physician and are only to be used within the Member State where it is produced. In addition, a competent authority must authorise hospital exemption for ATMPs and they must comply with the same national requirements concerning quality, traceability and pharmacovigilance that apply to authorised medicinal products.
Stakeholders present at the meeting called for transparency on the use of exemption products in the EU, so that industry, academia, regulators and even patients, can find out more easily which products are being used and for which indications. Specific proposals were for easily accessible registries and other electronic databases.
Subsequent to the meeting and the publication of the report, Anne Black, EAHP’s representative at the meeting, commented:
“Whilst discussion at the meeting did focus on potential flexibilities in application of Good Manufacturing Practice requirements in the area of ATMPs, I emphasised that healthcare professionals and patients have an expectation that any product provided to a patient is of appropriate safety, efficacy and quality. This should never be compromised.
On improving research and development of ATMPs, it is vital that clinical trial pharmacists are involved at an early stage as their expertise can greatly facilitate safe introduction.
Finally, careful consideration is needed to the availability of stability data for ATMPs as in my experience many trials require preparation in the clinical area due to the short expiry“.
More information here.
**EAHP EU Monitor readers with an interest in ATMP matters should be aware of two current public consultations in the policy area.
The Innovative Medicines Initiative (IMI) is conducting a consultation on what the principal barriers to ATMP uptake may be, and how these could be overcome. Stakeholder input is invited. The deadline is 25th July 2016. Consultation here.
In a separate consultation, the European Commission is seeking input to draft guidelines on Good Manufacturing Practice (GMP) for ATMPs. The consultation closes on 26th September 2016 and is available here.
 European Commission seeks experts to advise on effective ways of investing in health
European Commission seeks experts to advise on effective ways of investing in health
The European Commission is seeking individuals to help advise on effective ways of investing in health. The call closes on 26 July 2016.
Selected individuals will take part in a panel that will prepare opinions on new forms of healthcare models and the challenges health systems are confronted in Europe, with a view to ensuring that health systems are effective, accessible and resilient.
Examples of potential areas where the panel could be invited to give opinions include, but are not limited to: primary care, hospital care, integrated care models, pharmaceutical expenditure, research and development, disease prevention and health promotion, links with the social protection sector, cross-border issues, system financing, information systems, and health inequalities.
Members will be appointed for a period of three years, and may serve up to three consecutive terms.
More information here.
 EJHP: Inpatient prescribing systems used in NHS Acute Trusts across England, a managerial perspective
EJHP: Inpatient prescribing systems used in NHS Acute Trusts across England, a managerial perspective
The online first edition of the European Journal of Hospital Pharmacy (EJHP) has published an original article exploring chief pharmacists’ opinions and experiences with respect to electronic prescribing and medicines administration (EPMA) systems.
Article here.























 The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.

 EMA and stakeholders explore how to improve patient access to ATMPs
EMA and stakeholders explore how to improve patient access to ATMPs European Commission seeks experts to advise on effective ways of investing in health
European Commission seeks experts to advise on effective ways of investing in health EJHP: Inpatient prescribing systems used in NHS Acute Trusts across England, a managerial perspective
EJHP: Inpatient prescribing systems used in NHS Acute Trusts across England, a managerial perspective
