The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
Learn more about the Oath to Society
 Last autumn, the European Association of Hospital Pharmacists (EAHP) and the European Society of Clinical Pharmacy (ESCP) collaboratively developed and launched the “Oath to Society. To ensure that the Oath are used widely, EAHP and ESCP members have started to support translation efforts.
Last autumn, the European Association of Hospital Pharmacists (EAHP) and the European Society of Clinical Pharmacy (ESCP) collaboratively developed and launched the “Oath to Society. To ensure that the Oath are used widely, EAHP and ESCP members have started to support translation efforts.
The Oath to Society is a promise that the members of EAHP and ESCP make to patients and the public they serve, the healthcare professionals they interact with and the health systems they work in. The launch event in October 2021 also involved key stakeholders relevant to both clinical and hospital pharmacists. The panellists from the International Association of Mutual Benefit Societies, the European Patients’ Forum, the European Specialist Nurses Organisation, the European Hospital and Healthcare Federation and the European Union of Medical Specialists shared their positive experiences in working collaboratively with clinical and hospital pharmacists and showed why the Oath to Society is important to their individual organisations. Equal and affordable access to medicines, patient-centred care and cooperation were amongst the themes that the panellists noted during their debate.
Touching on trust and respect, different aspects of the patient care pathway, the multidisciplinary care team, disease prevention and health promotion, education and the future development of pharmacy practice, the Oath to Society is all-encompassing. To ensure their wide uptake as a compass for pharmacists to adhere to the highest standards of ethics, integrity and professionalism, EAHP and ESCP are working on translating the “Oath to Society” into as many languages as possible. So far, the following languages have been made available: Bosnian, Chinese, English, French, German, Hungarian, Nepalese, Persian, Polish, Portuguese and Slovak.
Learn more about the Oath to Society HERE
Contribute to the survey of EAHP’s SIG for the Investigation of Medication Errors in Intensive Care Units

Only a few days to contribute to the research activity of EAHP’s Interest Group (SIG) for the Investigation of Medication Errors in Intensive Care Units (ICUs) on medication error prevention strategies in intensive care settings across Europe. Healthcare professionals working in any intensive care setting involved with medicines and medication safety officers are encouraged to respond to the survey by the 8th of May.
Patient safety is a priority for healthcare organisations worldwide. Due to the complex nature of the ICU setting, specific strategies for improving medication safety are likely to be particularly important. EAHP’s SIG for the Investigation of Medication Errors in ICUs is consequently looking to identify medication error prevention strategies both in use and being planned in ICUs across Europe, in order to develop policy recommendations for medication safety improvement.
The survey has received ethical approval from University College London Research Ethics Committee and all information provided by survey participants will be confidential and the anonymity of participants will be protected throughout the study. Participation is completely voluntary and participants can stop completing the survey at any time. It is also possible to skip questions. It should take approximately 10 to 20 minutes to complete the survey. Feedback can be shared until the 8th of May 2022 via one of the seven different language versions of the survey (English, Estonian, French, German, Italian, Slovenian and Spanish).
EAHP’s SIG looks forward to receiving your input to the survey to identify examples of medication error prevention strategies in use and/or being planned in European ICUs.
Access the English version of the survey
https://easy-feedback.de/SIGSurveymedicationerrors/1449652/2x88c0
Access the Estonian version of the survey
https://easy-feedback.de/SIGsurveymedicationerrors/1453544/8LHzg3
Access the French version of the survey
https://easy-feedback.de/SIGMedicationerrorsFrenchtranslation/1453156/7hV7uR
Access the German version of the survey
https://easy-feedback.de/SIGsurveymedicationerrorsGermantranslation/1453554/vqStyC
Access the Italian version of the survey
https://easy-feedback.de/SIGsurveymedicationerrorsItaliantranslation/1453682/Nq2UwR
Access the Slovenian version of the survey
https://easy-feedback.de/s/1453648/Bb28RA
Access the Spanish version of the survey
https://easy-feedback.de/SIGsurveymedicationerrorspanishtranslation/1453331/z7Qec9
Join the focus group interviews of EAHP’s SIG for the Investigation of Medication Errors in Intensive Care Units
 EAHP’s Special Interest Group for the Investigation of Medication Errors in Intensive Care Units (ICU) would like to invite you to take part in an online focus group interview.
EAHP’s Special Interest Group for the Investigation of Medication Errors in Intensive Care Units (ICU) would like to invite you to take part in an online focus group interview.
The focus group interview is a part of extensive study composed of a literature review, a survey and focus group interviews on medication error prevention strategies and patient safety culture within the ICU environment across Europe. The language used in the focus group interview will be English.
The aim of the focus group is to explore the experiences of healthcare professionals of patient safety culture and medication error prevention strategies in intensive care settings across Europe. We hope that the information we get, along with other research we are conducting, can be used to develop policy recommendations for medication safety improvement in ICUs across Europe. This research has received ethical approval from the Ethical Review Board in the Humanities and Social and Behavioural Sciences, University of Helsinki (18/2022, 18.3.2022).
Healthcare professionals working in any intensive care setting involved with medicines or in any medication safety role can take part in this study.
Participation in this research is completely voluntary, and all the information you provide will be kept confidential. All data collected will be pseudonymised, meaning that any data identifying participants will be removed before the analyses. Please read the attached information sheet on the study which provides further detail on the research.
If you are interested in taking part in this research, please email the Principal Investigator, Adjunct Professor Raisa Laaksonen (raisa.laaksonen@helsinki.fi), University of Helsinki, Finland, co-chair of the EAHP Special Interest Group by the 10th of May 2022.
The focus group interview will be arranged using an online videoconferencing facility on
- Friday, 13th of May (12.00 to 14.00 CET)
- Tuesday, 17th of May (13.00 to 15.00 CET)
- Wednesday, 18th of May (12.00 to 14.00 CET)
- Monday, 23rd of May (12.00 to 14.00 CET)
Thank you for considering to take part in this research!
Access the Participant Information Sheet HERE
Access the Consent Form HERE
European Health Data Space proposal launched
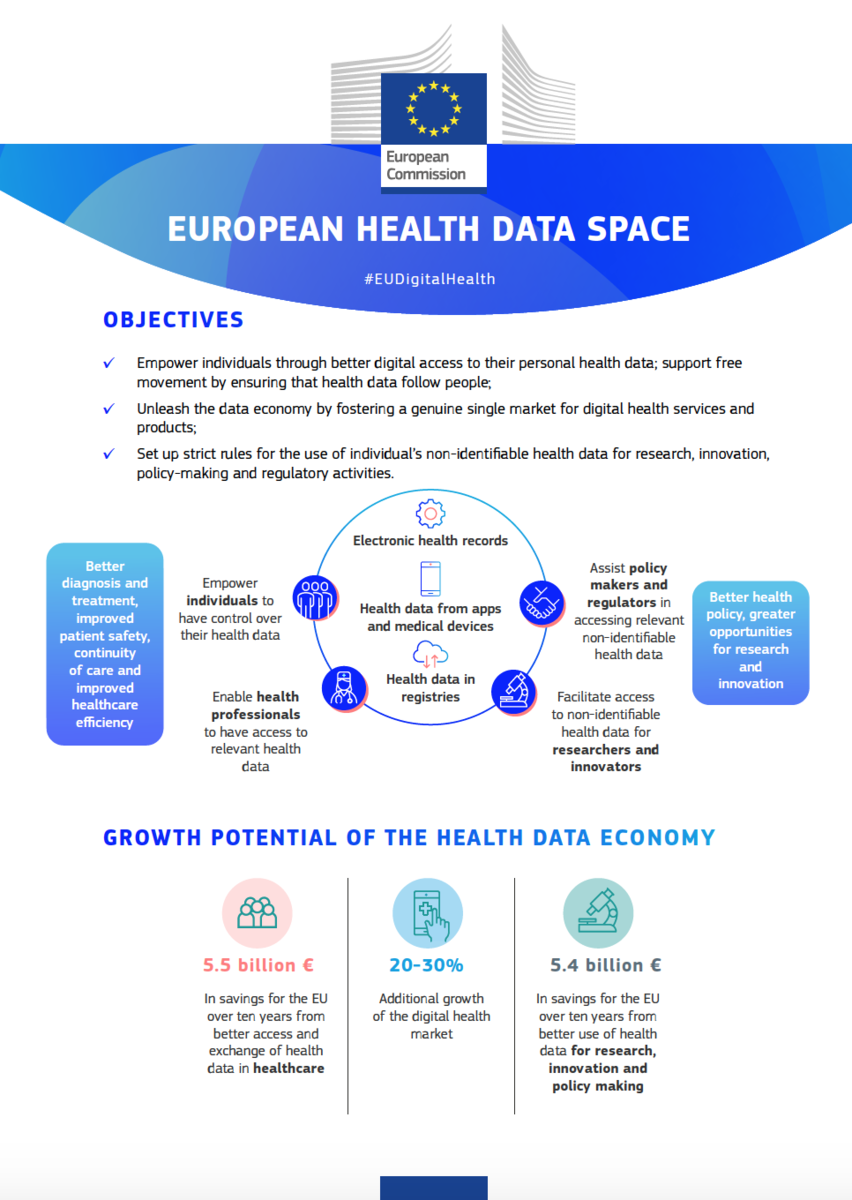 Yesterday, the European Commission published the proposal for the European Health Data Space (EHDS) that seeks to revolutionise healthcare provision in Europe by empowering people to control and utilise their health data, fostering a genuine single market for digital health services and offering a consistent, trustworthy and efficient framework to use health data for research, innovation, policy-making and regulatory activities, while ensuring full compliance with the EU’s high data protection standards.
Yesterday, the European Commission published the proposal for the European Health Data Space (EHDS) that seeks to revolutionise healthcare provision in Europe by empowering people to control and utilise their health data, fostering a genuine single market for digital health services and offering a consistent, trustworthy and efficient framework to use health data for research, innovation, policy-making and regulatory activities, while ensuring full compliance with the EU’s high data protection standards.
The EHDS is a building block for a strong European Health Union and it interlinks with the General Data Protection Regulation (GDPR), the Data Governance Act and the Data Act. For healthcare professionals, the EHDS proposal includes faster access to patients’ health records, including across borders and easier access to health records from different systems, greatly reducing administrative burdens. The proposal that has been put forward by the European Commission will now be discussed by the Council and the European Parliament.
Learn more about the EHDS proposal HERE
EHMA workshop: Everybody’s business: value-based healthcare
 The European Health Management Association (EHMA) will be hosting the executive workshop ‘Everybody’s business: value-based healthcare’ digitally on 10 May from 14:00 to 18:00 CET. This is the last workshop in the series ‘The many avenues of health management’.
The European Health Management Association (EHMA) will be hosting the executive workshop ‘Everybody’s business: value-based healthcare’ digitally on 10 May from 14:00 to 18:00 CET. This is the last workshop in the series ‘The many avenues of health management’.
The workshop will look at what value-based healthcare is and how health systems and organisations can make it a reality. It has been designed for all protagonists of change in the management and transformation of health systems.
More information about the workshop can be found HERE
EJHP: The May issue is available!
 The newest issue of the European Journal of Hospital Pharmacy (EJHP) is out. The editorial focuses on medicine packaging pictograms in the context of the electronic product information (ePI) proposal. Some of the original research articles provide insights on reducing the risk of non-sterility of aseptic handling in hospital pharmacies, the discontinuation of cholinesterase inhibitor treatment and Omega-3 for the prevention of cardiovascular diseases. Two systematic reviews and one protocol are also included.
The newest issue of the European Journal of Hospital Pharmacy (EJHP) is out. The editorial focuses on medicine packaging pictograms in the context of the electronic product information (ePI) proposal. Some of the original research articles provide insights on reducing the risk of non-sterility of aseptic handling in hospital pharmacies, the discontinuation of cholinesterase inhibitor treatment and Omega-3 for the prevention of cardiovascular diseases. Two systematic reviews and one protocol are also included.
Read the May issue HERE
[EAHP Statement Corner]
Have you met EAHP’s Statement Implementation Ambassadors?
To promote the implementation of the European Statements of Hospital Pharmacy at the national level EAHP has teamed up with motivated and enthusiastic hospital pharmacists willing to help their countries and others move towards Statement Implementation, the so-called “Implementation Ambassadors”. They act as the primary link between EAHP, their national associations and the implementation activities done within their countries. In case you are curious about the Implementation Ambassador working in your country you can find out more about him/her via the Statement website.
[Spotlight]
EAHP Position Paper on Hospital Pharmacy Specialisation
Hospital pharmacists are the key stakeholders responsible for medication management and safety in the hospital environment, covering both in- and out-patient services and supporting the seamless transition of care for patients moving within the healthcare system. To provide the best treatment for all patients, hospital pharmacists must be able to operate in a complex hospital setting and work collaboratively within multi-disciplinary healthcare teams.
To prepare the hospital pharmacy profession for the future, the European Association of Hospital Pharmacists (EAHP) adopted the European Statements of Hospital Pharmacy in 2014. They express commonly agreed objectives that every European health system should aim for in the delivery of hospital pharmacy services. To further enhance the quality, safety and equity of access to patient care in every European country, EAHP additionally created the Common Training Framework (CTF) project for hospital pharmacy education in Europe. This project not only fosters the further development of hospital pharmacy practice but also seeks to guarantee the access of European citizens to the highest available standard of care and the freedom of movement of the hospital pharmacy profession which is currently not accessible to all.
In June 2021, EAHP’s General Assembly adopted a Position Paper on Hospital Pharmacy Specialisation. The position centres around advancing the profession by harmonising the recognition of hospital pharmacy education, enhancing the role of the hospital pharmacist and preparing the profession for future challenges.
To make a difference in medication by advancing the hospital pharmacy profession, EAHP
- calls on the European Commission and the Member States to assist the Association in setting up a CTF through the adoption of a delegated act;
- touches on the need for Member States to recognise the changing role of the hospital pharmacists and further foster their implementation; and,
- underlines the importance of further promoting the uptake of such cross-sector tools inter-sector communication, coordination and multi-disciplinary collaboration in all healthcare facilities should be strengthened.
To adequately address future challenges linked to the ageing society, changing healthcare needs and other unknown factors, like future pandemics, EAHP urges that Member States invest in better workforce planning for the hospital pharmacy profession, including the availability of hospital pharmacy services for all patients of each hospital.
Read EAHP’s Position Paper on Hospital Pharmacy Specialisation HERE
























 Last autumn, the European Association of Hospital Pharmacists (EAHP) and the European Society of Clinical Pharmacy (ESCP) collaboratively developed and launched the “Oath to Society. To ensure that the Oath are used widely, EAHP and ESCP members have started to support translation efforts.
Last autumn, the European Association of Hospital Pharmacists (EAHP) and the European Society of Clinical Pharmacy (ESCP) collaboratively developed and launched the “Oath to Society. To ensure that the Oath are used widely, EAHP and ESCP members have started to support translation efforts.
 EAHP’s Special Interest Group for the Investigation of Medication Errors in Intensive Care Units (ICU) would like to invite you to take part in an online focus group interview.
EAHP’s Special Interest Group for the Investigation of Medication Errors in Intensive Care Units (ICU) would like to invite you to take part in an online focus group interview. Yesterday, the European Commission published the proposal for the European Health Data Space (EHDS) that seeks to revolutionise healthcare provision in Europe by empowering people to control and utilise their health data, fostering a genuine single market for digital health services and offering a consistent, trustworthy and efficient framework to use health data for research, innovation, policy-making and regulatory activities, while ensuring full compliance with the EU’s high data protection standards.
Yesterday, the European Commission published the proposal for the European Health Data Space (EHDS) that seeks to revolutionise healthcare provision in Europe by empowering people to control and utilise their health data, fostering a genuine single market for digital health services and offering a consistent, trustworthy and efficient framework to use health data for research, innovation, policy-making and regulatory activities, while ensuring full compliance with the EU’s high data protection standards. The European Health Management Association (EHMA) will be hosting the executive workshop ‘Everybody’s business: value-based healthcare’ digitally on 10 May from 14:00 to 18:00 CET. This is the last workshop in the series
The European Health Management Association (EHMA) will be hosting the executive workshop ‘Everybody’s business: value-based healthcare’ digitally on 10 May from 14:00 to 18:00 CET. This is the last workshop in the series  The newest issue of the European Journal of Hospital Pharmacy (EJHP) is out. The editorial focuses on medicine packaging pictograms in the context of the electronic product information (ePI) proposal. Some of the original research articles provide insights on reducing the risk of non-sterility of aseptic handling in hospital pharmacies, the discontinuation of cholinesterase inhibitor treatment and Omega-3 for the prevention of cardiovascular diseases. Two systematic reviews and one protocol are also included.
The newest issue of the European Journal of Hospital Pharmacy (EJHP) is out. The editorial focuses on medicine packaging pictograms in the context of the electronic product information (ePI) proposal. Some of the original research articles provide insights on reducing the risk of non-sterility of aseptic handling in hospital pharmacies, the discontinuation of cholinesterase inhibitor treatment and Omega-3 for the prevention of cardiovascular diseases. Two systematic reviews and one protocol are also included.