THE RISK MANAGEMENT OF THE PHARMACY PREPARATIONS IN THE HOSPITAL PHARMACIES (submitted in 2019)
Pdf

European Statement
Production and Compounding
Author(s)
ADRIANA DURCANSKA
Why was it done?
The quality and safety standards of pharmacy preparations are not harmonised throughout Europe. They fall under the national competencies of individual European countries.
What was done?
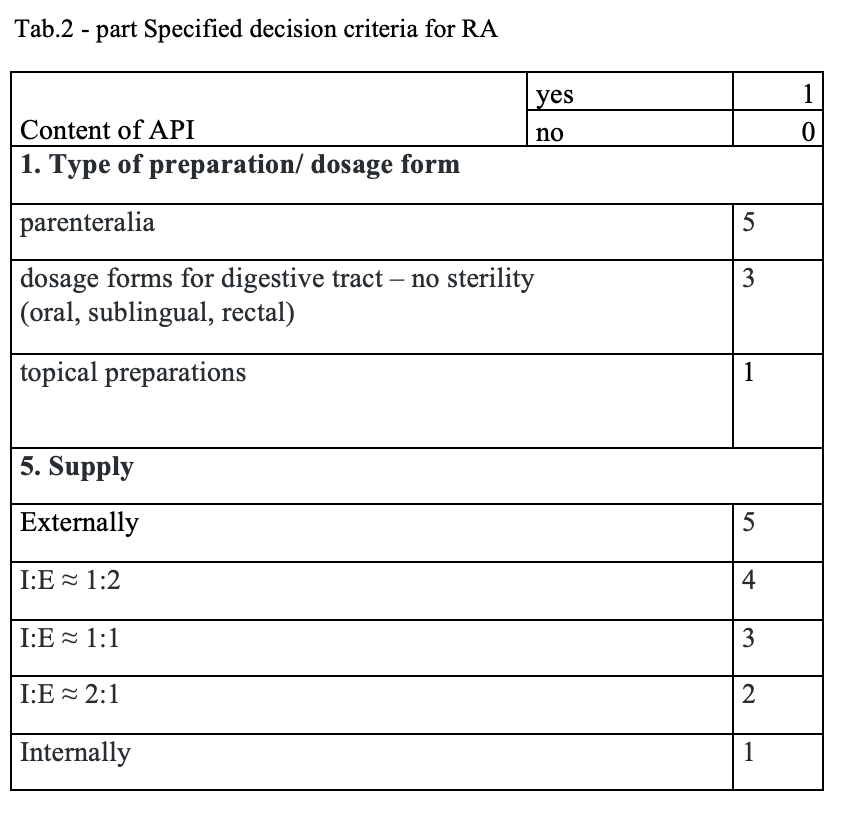
The quantitative risk assessment of the pharmacy preparations for stock in hospital pharmacies (HPs) in accordance with Resolution EDQM CM / Res (2016) 1; to specify the decision criteria for the risk assessment; the risk management of the pharmacy preparations for stock in the country; to design a check list of the risk assessment for extempore preparations.
How was it done?
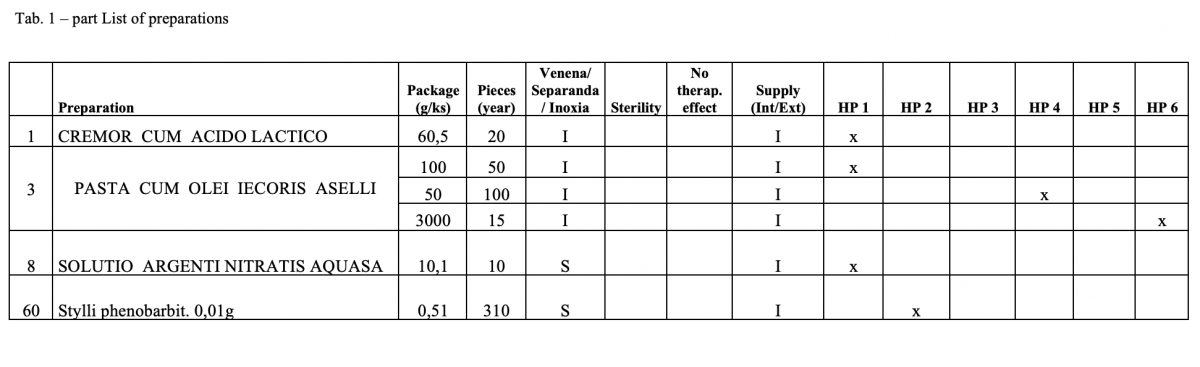
Out of the total number of 53 hospital pharmacies contacted, 5 pharmacies sent a suitable file.
What has been achieved?
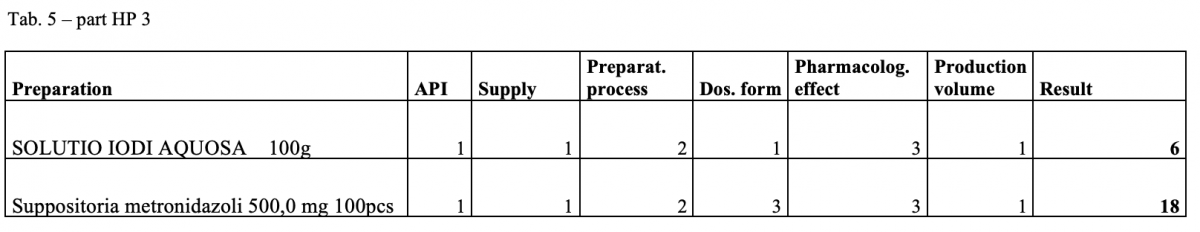
A total of 170 types of medicines are being prepared in HPs. One HP had the result of the risk ≥ 100 when preparing ophthalmic medicines. Annex A is a check list designed to assess the risk of extempore preparations.
What next?
The management is and will be forced to consider its introduction or to use another model: hospital – GMP / outsourcing / central pharmacy preparing and distributing. The aim of using the document in hospital pharmacies of the country.



























