 The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
You can subscribe to receive the EAHP EU Monitor by email HERE.
 Organisations and individuals invited to register for common training framework consultation
Organisations and individuals invited to register for common training framework consultation
The European Association of Hospital Pharmacists (EAHP) is inviting all interested organisations and individuals to register to participate in an online consultation on a common training framework (CTF) for hospital pharmacy.
A common training framework for hospital pharmacy education in Europe will represent an agreement on the competencies, knowledge, skills and attitudes required by the profession in order to deliver the 44 European Statements of Hospital Pharmacy. These statements represent a vision for hospital pharmacy practice development in all countries for the purpose of improving patient care.
The consultation on the framework’s initial content will be conducted according to Delphi principles. The online portal will enable contributors to participate anonymously and continuously, as well as be informed by the ongoing contributions of others. Consecutive rounds of consultation, facilitated by an independent moderator team, will aim to achieve a consensus on the content in time to be voted on at the EAHP annual General Assembly in Malta in June 2017.
The registration portal for the consultation will close on 31 January 2017, with the first round of consultation commencing in February 2018.
Joan Peppard, EAHP President, remarked:
“Transparency and wide consultation are fundamental to the common training framework. In the process of finalising the content of a common training framework for hospital pharmacy we want to ensure all perspectives are drawn upon in order to enrich the outcome. The perspectives we are searching for include those from different national health systems, the various healthcare professionals that hospital pharmacists work with, system managers, education providers, other branches of pharmacy, and, of course, the patients we serve. The more we collaborate in the process of creation, the stronger the result will be. On behalf of hospital pharmacy, I want to convey encouragement to ALL interested individuals and organisations to register to participate today!“
The registration portal for the CTF consultation is available HERE
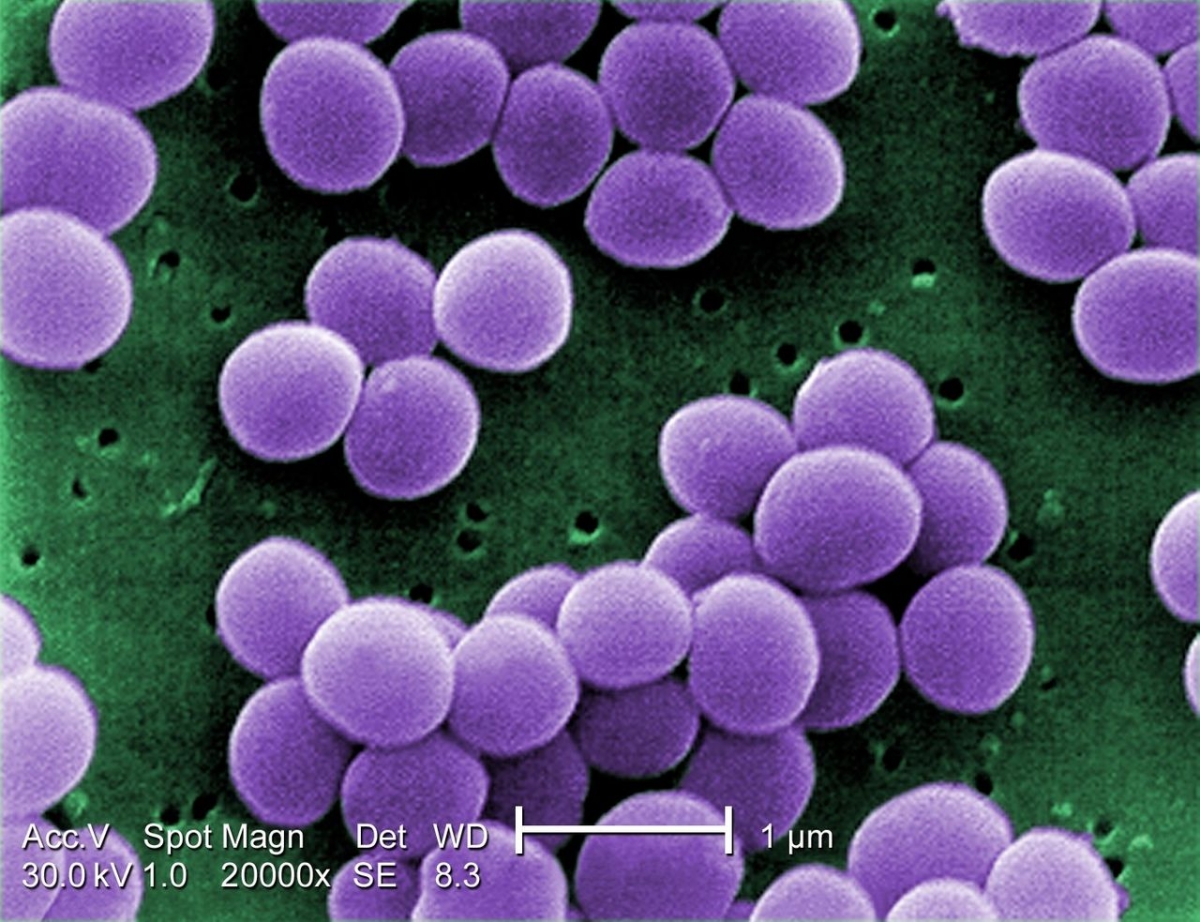
Alarming New ECDC Report confirms last line antibiotics are failing
The European Centre for Disease Control and Prevention has published its latest EU-wide data on antibiotic resistance and antibiotic consumption. In 2015, antibiotic resistance continued to increase for most bacteria and antibiotics under surveillance.
In particular, the EU average percentage of carbapenem resistance in Klebsiella pneumoniae increased from 6.2% in 2012 to 8.1% in 2015, and combined resistance to carbapenems and polymyxins (e.g. colistin) was sometimes reported. These two groups of antibiotics are considered last-line antibiotics as they usually are the last treatment options for patients infected with bacteria resistant to other available antibiotics.
While antibiotic consumption in hospitals significantly increased in several EU Member States, antibiotic consumption in the community decreased in six EU Member States.
ECDC Acting Director, Dr Andrea Ammon, said: “Antibiotic resistance in Klebsiella pneumoniae is of increasing concern in Europe. More than one third of the isolates reported to ECDC for 2015 were resistant to at least one of the antibiotic groups under surveillance, and combined resistance to multiple antibiotic groups was common. Moreover, the emergence of K. pneumoniae infections with combined resistance to carbapenems and colistin is worrisome and an important warning that options for treatment are now even more limited than in the past“.
Dr Ammon added: “However, the decrease of antibiotic consumption in the community in six countries is a positive sign and shows that we are starting to use antibiotics more prudently. Prudent use of antibiotics is pivotal, both in the community and in hospitals, to ensure that these drugs remain effective“.
ECDC’s data also show that antibiotic resistance in Escherichia coli, one of the most frequent causes of bloodstream infections and community- and healthcare-associated urinary tract infections, requires close attention as the percentages of isolates resistant to commonly used antibiotics continues to increase throughout Europe. In contrast, the percentage of meticillin-resistant Staphylococcus aureus (MRSA) showed a significantly decreasing trend at EU/EEA level between 2012 and 2015. Despite this positive development, MRSA remains a public health priority as eight out of thirty countries reported percentages above 25%.
More information HERE

European Commission launch consultation on Paediatric Medicine Regulations
The European Commission is inviting stakeholders to provide comment on how prevailing EU regulations related to paediatric medicine development might be improved. The deadline for comments is 20th February 2017.
2017 will mark ten years since the coming into being of the 2007 Paediatric Regulation. This piece of EU law aimed to improve the regulatory environment for paediatric medicine development by increasing obligations upon companies to include ‘paediatric investigation plans‘ within their processes for conducting research on potential new medicines. These tougher obligations are also complimented by incentives for paediatric research such as extended market exclusivity and free scientific advice.
The document accompanying the consultation acknowledges that the 2007 Paediatric Regulation undoubtedly improved attention on paediatric development, with nearly 1,000 paediatric investigation plans agreed as a result. However, other areas of the regulation may not have been so successful in their intent.
A series of 17 prompting questions aims to draw out from stakeholders their experiences of the regulation, and suggestions for improvement. More information HERE

EJHP: Intrathecal cyclodextrin in the treatment of Niemann-Pick disease type C
The online first edition of the European Journal of Hospital Pharmacy (EJHP) has published a new case report examining experience of intrathecal hydroxypropyl-β-cyclodextrin therapy for a child with Niemann-Pick disease type C.
More information HERE
























 The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe. Organisations and individuals invited to register for common training framework consultation
Organisations and individuals invited to register for common training framework consultation


