EU Monitor - 13th October 2021 - Learn more about EAHP’s Special Interest Groups
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
EAHP's Special Interest Groups – a new way of engaging the voices of hospital pharmacists

At the beginning of this year, the European Association of Hospital Pharmacists (EAHP) opened a new chapter of hospital pharmacy engagement by setting up Special Interest Groups (SIGs). EAHP's SIGs gather and evaluate the evidence in specific, innovative and novel fields of hospital pharmacy practice with the aim to address patient needs and to advance the profession.
Three of EAHP’s SIGs have already started their work. The SIG on Hazardous Medicinal Products (financially supported by Amgen) is carrying out an investigation into the classification systems that are used throughout Europe for hazardous medicinal products. Based on their findings and discussion outcomes the SIG is developing recommendations and guidance to support hospital pharmacists and their advocacy work. EAHP’s Special Interest Group for the Investigation of Medication Errors in Intensive Care Units (financially supported by BD) seeks to determine the prevalence of medication errors in intensive care units, their causes or contributing factors, and strategies to improve medication safety and prevent medication errors. The findings of this group will be published in form of a report and policy recommendations towards the end of next year.
EAHP’s third SIG on Automated Medication Management (financially supported by Omnicell) is tasked with investigating the potential benefits of automation in the hospital setting in terms of patient safety, length of stay and staff efficiency for patient care and medication inventory management. Particular focus will be put on addressing how automation in dispensing can help achieve one of the key objectives of the World Health Organisation – reducing medication errors.
A fourth SIG – focused on the Use of Prefilled Syringes in Intensive Care Units and Operating Rooms (financially supported by BD) – will be kicking off its activities in November 2021. The work of this SIG will follow three main objectives:
- Gathering evidence on the risks associated with the use of vials vs the use of prefilled syringes for patient safety in intensive care and operating room settings within EAHP member states.
- Evaluating existing health economic model tools to determine starting points
- Evidence generation of existing models and/or new models in member states to compare added benefits and associated costs when using prefilled syringes versus vials/ampoules in intensive care and operating room settings
Hospital pharmacists and other healthcare professionals interested in joining this group can reach out to info[at]eahp[dot]eu to obtain the call for the expression of interest.
More information about the SIG on the Hospital pharmacist’s preparedness for rAAV GTx (financially supported by Pfizer) and the SIG focused on Eliminating avoidable harm within the supply, preparation and safe administration of injectable medicines in Europe (financially supported by Baxter) will pick up their activities in the coming weeks. Call for the expression of interest for these two groups will be shared shortly with EAHP’s member associations.
SIGs bring together groups of professionals with an interest in advancing a specific area of knowledge, learning or technology in order to improve practice and define potential solutions within a particular area. Membership focuses on hospital pharmacists but also includes other experts such as physicians, nurses, pharmaceutical industry leaders and patient representatives.
If you want to learn more about EAHP’s SIGs or have questions, contact us at info[at]eahp[dot]eu
EAHP-EPSA Student Science Award – share your research!
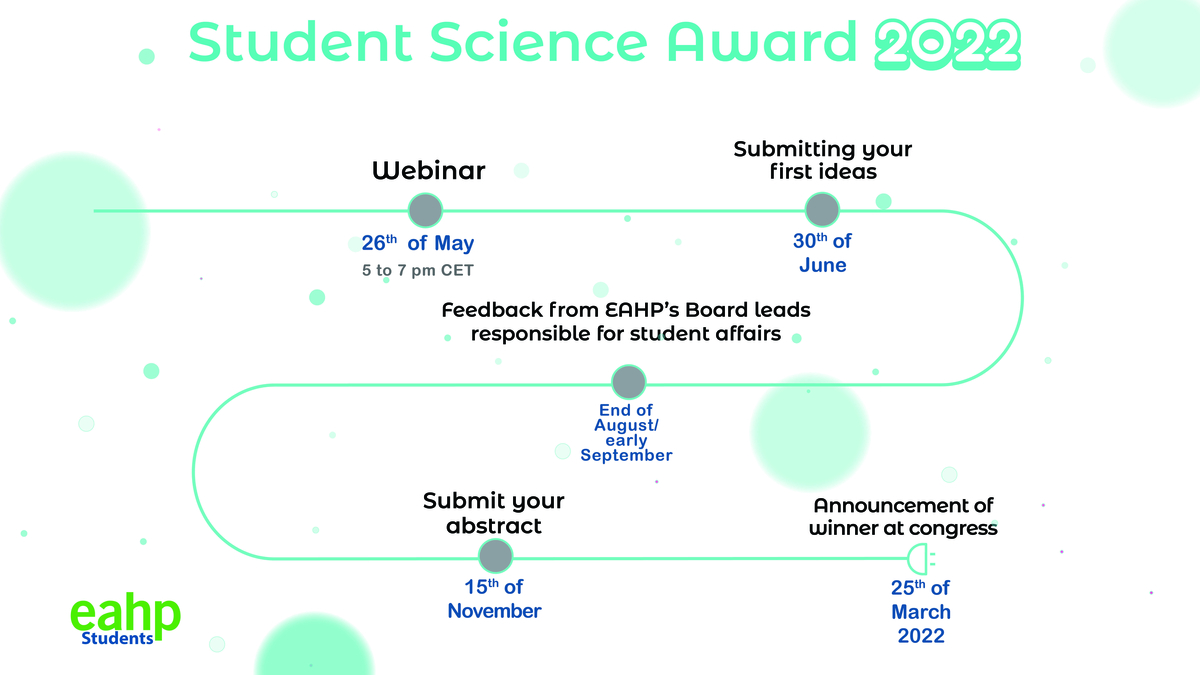
The next edition of the EAHP-EPSA Student Science Award is in full swing. Students and recent graduates only have until the 15th of November 2021 to submit their abstracts for the competition. The author of the winning abstract will receive a complimentary registration to the 26th Congress of EAHP, including the coverage of travel and accommodation expenses (up to 500 Euros), as well as official recognition at the EAHP Congress closing ceremony on the 25th of March 2022.
The EAHP-EPSA Student Science Award recognises and honours the best scientific research authored by a pharmacy student or recent graduate. Since 2011 it is offered to one member of the European Pharmaceutical Students' Association (EPSA) who has conducted research in the field of hospital and/or clinical pharmacy. Students are encouraged to submit their research to enter into the competition. The abstracts are reviewed in accordance with the criteria of innovation, originality, and contribution to the development of hospital pharmacy. If you want to join the largest European hospital pharmacy congress taking place in Vienna, Austria next year, don’t wait too long and start finalising your abstract.
More information about the EAHP-EPSA Student Science Award and the submission procedure HERE
EMA hosts Clinical Trials Information System (CTIS) virtual information day on the 26th of October 2021

On the 26th of October 2021 from 13:15 to 17:00 (CET), the European Medicines Agency (EMA) will be hosting a virtual information day in liaison with DIA for the Clinical Trials Information System (CTIS). The aim of the event is to help future CTIS users prepare for submitting and managing clinical trial applications in CTIS after its go-live on 31 January 2022. The event is aimed at clinical trial sponsors, contract research organisations (CRO), staff at national competent authorities and ethics committee members.
EMA’s virtual information day will present a high-level overview of CTIS via live demonstration of the system's restricted workspaces. It will also present the future user’s perspective on preparing for CTIS go-live, with presentations by future sponsors and Member State users of CTIS. The event will provide ample time for the audience to ask questions.
Learn more about the event and how to register HERE
ECDC builds study infrastructure to monitor COVID-19 vaccine effectiveness

The European Centre for Disease Prevention and Control (ECDC) is creating new infrastructure to allow for regular monitoring and analysis of COVID-19 vaccine effectiveness over time, using a multi-country approach, through the implementation of studies in different settings. One of these settings is hospitals involving more than 30 in 10 different EU countries, where the primary aim is to assess vaccine effectiveness against severe disease.
On October 8, ECDC published an initial interim analysis presenting pooled estimates of vaccine effectiveness against severe acute respiratory infection due to laboratory-confirmed SARS-CoV-2 in hospitalised patients. The report indicates a high vaccine effectiveness of 90% when assessed 14 days after a full vaccination course, and for vaccines that received conditional marketing authorisation by the European Medicines Agency (EMA). Data were collected between December 2020 and June 2021 and indicates high efficacy in preventing hospitalisation for COVID-19. Regular updates of these results will be provided periodically as the study continues, including more extensive analyses to assess factors that may affect vaccine effectiveness, such as variants and length of time since vaccination. The outcome of this study will contribute to knowledge on the performance of COVID-19 vaccines in real-life settings.
Read the interim analysis HERE
Questionnaire: Ward pharmacists in oncology

As part of her PhD project in clinical pharmacy, a member of the German Society of Hospital Pharmacists has put together a survey in order to demonstrate the benefits of a ward pharmacist in oncology and thus help to increase the patient's therapeutic safety. The project puts a specific focus on interprofessional collaboration of ward pharmacists, doctors and nursing in oncology.
To assess the impact of possible improvements and to get the most comprehensive picture possible, it is necessary to determine the current situation throughout Europe. For representative data collection, only one answer from each hospital pharmacy is allowed. Your information will be handled strictly confidential and the results will only be published anonymously. Responses can be shared until 15 December 2021. Participation only takes a few minutes and aims also at supporting pharmacists in clinics without a cytostatics department.
Access the survey via the following LINK
EJHP: Evaluation of amikacin dosing schedule in critically ill elderly patients with different stages of renal dysfunction

Amikacin is still a widely used aminoglycoside for the treatment of life-threatening infections. This study aimed to characterise the pharmacokinetics of amikacin in critically ill elderly patients with renal dysfunction, and to evaluate if the available dose adjustment schedules dependent on renal function would be appropriate for empirical dosing. Critically ill patients aged >60 years with a creatinine clearance of >20 mL/min in need of treatment with amikacin were randomly enrolled. All the patients received approximately 25 mg/kg amikacin. The outcome of the study showed that the initial dosing of amikacin in critically ill elderly patients should not be reduced, even in the context of renal impairment. Regarding the dose adjustment in renal impairment, dosing intervals estimation, no decision can be made based on the creatinine clearance and the first dose individualisation method in terms of the two-sample measurements may be considered as an appropriate strategy.
Read the article HERE

[EAHP Statement Corner]
Have you completed your SAT yet?
To support the implementation of the European Statements of Hospital Pharmacy, EAHP has developed a self-assessment tool (SAT) that helps you understand the level of Statement implementation in your pharmacy. The tool has been rolled out across Europe. Different language versions (Czech, English, French, German, Greek, Hungarian, Italian, Polish, Portuguese, Romanian, Serbo-Croatian, Spanish and Turkish) have been made available to enable as many hospital pharmacists as possible to use the tool. Talk to your chief pharmacist and encourage him/her to work with the SAT. In case you are the head of the pharmacy get your team together and complete your assessment today with the help of the SAT.
Learn more about SAT HERE
[Covid-19 Updates]
EAHP’s COVID-19 Resource Centre
To assist its member associations and individual hospital pharmacists in this critical time with the provision of the best possible care for patients, EAHP has decided to gather and make available information on COVID-19 relevant for the hospital pharmacy profession.
Access the Resource Centre HERE

[Spotlight]
EAHP Position Paper on Access to Medicines
Ensuring that patients receive the medication they need to improve their health and to prevent and cure diseases is the key topic of EAHP's Position Paper on Access to Medicines. Given that growing healthcare expenditure has become a problem for many European countries that also affects patients who are increasingly faced with avoidable accessibility and affordability issues, EAHP has chosen to look at the barriers to access and enablers that could help improve it.
Lack of purposeful procurement practices, national pricing and reimbursement policy choices jeopardising patients' adequate access, medicine shortages and the unavailability in certain markets that are leading to inequity between Member States are the main barriers identified in the position. For the provision of affordable medicines of good quality that are given in a timely manner to patients, EAHP advocates for breaking down these barriers to treatment access and calls for uptake of enablers that promote and safeguard the access of patients to both new life-saving medicines and older, essential medicines must be increased. Enablers mentioned refer to health technology assessments (HTAs), including common reports at EU level, collaboration and best practice sharing on pricing and reimbursement, increasing the use of prevention measures and fostering innovation and research
To achieve an equilibrium between the barriers and the enablers to treatment access, the following has been put forward by EAHP:
- Recommendation to leverage and utilise the expertise of the hospital pharmacist in pharmacoeconomics and the assessment of drug effectiveness within value-based evaluation approaches. Additionally, the implementation of the forthcoming HTA Regulation should be used for the expansion of healthcare professional input in HTAs at both European and national level.
- Support for EURIPID and the suggestion that the tool developed by this collaboration should not only be applied on its own but in conjunction with other policy measures, including transparency.
- Collaboration between hospital managers and hospital pharmacists to increase the uptake of risk assessments in hospitals.
- Investments to support the development of innovative proposals and the encouragement of practice-based research projects to investigate new fields of infectious disease control such as immunotherapy and to optimise the cost-effectiveness of systems for surveillance on antibiotic use and resistance.
In relation to the developments linked to the European Health Union aided by the implementation of the Pharmaceutical Strategy, EAHP underlined its commitment to working together with the European institutions and other stakeholders by giving a voice to access issues that otherwise might be forgotten.
Read the Position Paper on Access to Medicines HERE
[Consultation]
European Commission – Public consultation on the revision of the general pharmaceutical legislation
This public consultation aims to collect views of stakeholders and the general public in order to support the evaluation of the existing general pharmaceutical legislation and the impact assessment of its revision. It builds further on the public consultation conducted for the preparation of the pharmaceutical strategy for Europe.
Deadline – 21st December 2021
Access the consultation HERE




