3 November - Discover the EU's Medicines Shortage Action Plan
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
Adapting EMA's New Mandate: Hospital Pharmacists' and Pharmacy Managers Data Sharing Survey

EHMA and EAHP are conducting a survey targeting hospital pharmacists and pharmacy managers, focusing on the collection and sharing of data related to medication demand and stocks. The survey aims to identify how hospitals currently gather and share data on medication stock and demand, identify any barriers to collecting information on medicines stocks, and explore the workload implications and digitalisation requirements for hospital pharmacy workflows in relation to data collection. The survey will remain open for three months. The results will be collected and published in a briefing paper that will be shared with EU policymakers and the European Medicines Agency.
Find the survey HERE.
5th EAHP Synergy Certification Course

The European Association of Hospital Pharmacists (EAHP) and SEFH (Spanish Society of Hospital Pharmacists – Sociedad Española de Farmacia Hospitalaria) are holding the 5th EAHP Synergy Certification Course on the 23rd and 24th of November, taking place in Alicante, Spain. Those who decide to join at short notice can still register for the event until November 11.
During this EAHP-SEFH Synergy Certification Course, attendees will be able to acquire the latest knowledge around the theme "Addressing outpatient pharmaceutical care needs from the perspective of hospital pharmacy". A variety of topics will be addressed in the Synergy Certification Course, including strategies developed by the different European countries for outpatient pharmaceutical attention improvement,development and establishment of healthcare stratification, best practices in patients with specific disease patterns (oncohematology, immune-mediated, neurodegenerative diseases, viral pathologies, pneumology, etc.), hospital pharmacy integration strategies in multidisciplinary teams of outpatient assistance, results of pharmaceutical care technology support initiatives in Spain, and agreement on the next steps for the expansion of the ground-breaking strategies in Spain and Europe.
You can find the full programme HERE, and registration can be done by clicking HERE.
Communication of the Commission on Addressing Medicine Shortage in the EU

The European Commission has published a communication to address critical medicine shortages in Europe. Together with EMA, they will launch a European Voluntary Solidarity Mechanism for medicines in October 2023. They will establish a European Union list of critical medicines by the end of 2023 and analyse selected medicines' supply chains by April 2024. Furthermore, they will aim for a Joint Action in 2024, which allows Member states to use regulatory exemptions to allow medicines to reach patients promptly, including extending the shelf-life and authorising alternatives. In addition, the Commission aims to publish an EU guidance on procuring medicines by early 2024 and opt for a joint procurement for antibiotics and respiratory virus treatments next winter. A Critical Medicines Alliance will be launched by early 2024 to enhance collaboration, coordinate EU-level public procurement, diversify global supply chains, and streamline funding. Lastly, a unified strategy for medicines stockpiling will be developed with Member States by the first half of 2024.
Learn more about it HERE.
Introduction to the European Voluntary Solidarity Mechanism to combat critical shortages
 The Executive Steering Group on Shortages and Safety of Medicinal Products (MSSG) of the European Medicines Agency (EMA) is developing a European Voluntary Solidarity Mechanism for medicines shortages together with the European Commission. This Mechanism allows a Member State that faces a critical shortage to request assistance from other Member States in obtaining medicine stocks. The Mechanism has strict conditions and should only be used as a last resort. More details on the Solidarity Mechanism can be found in the MSSG toolkit published by EMA.
The Executive Steering Group on Shortages and Safety of Medicinal Products (MSSG) of the European Medicines Agency (EMA) is developing a European Voluntary Solidarity Mechanism for medicines shortages together with the European Commission. This Mechanism allows a Member State that faces a critical shortage to request assistance from other Member States in obtaining medicine stocks. The Mechanism has strict conditions and should only be used as a last resort. More details on the Solidarity Mechanism can be found in the MSSG toolkit published by EMA.
Learn more about it HERE.
Together, we tackle AMR: European Antibiotic Awareness Day (EAAD)
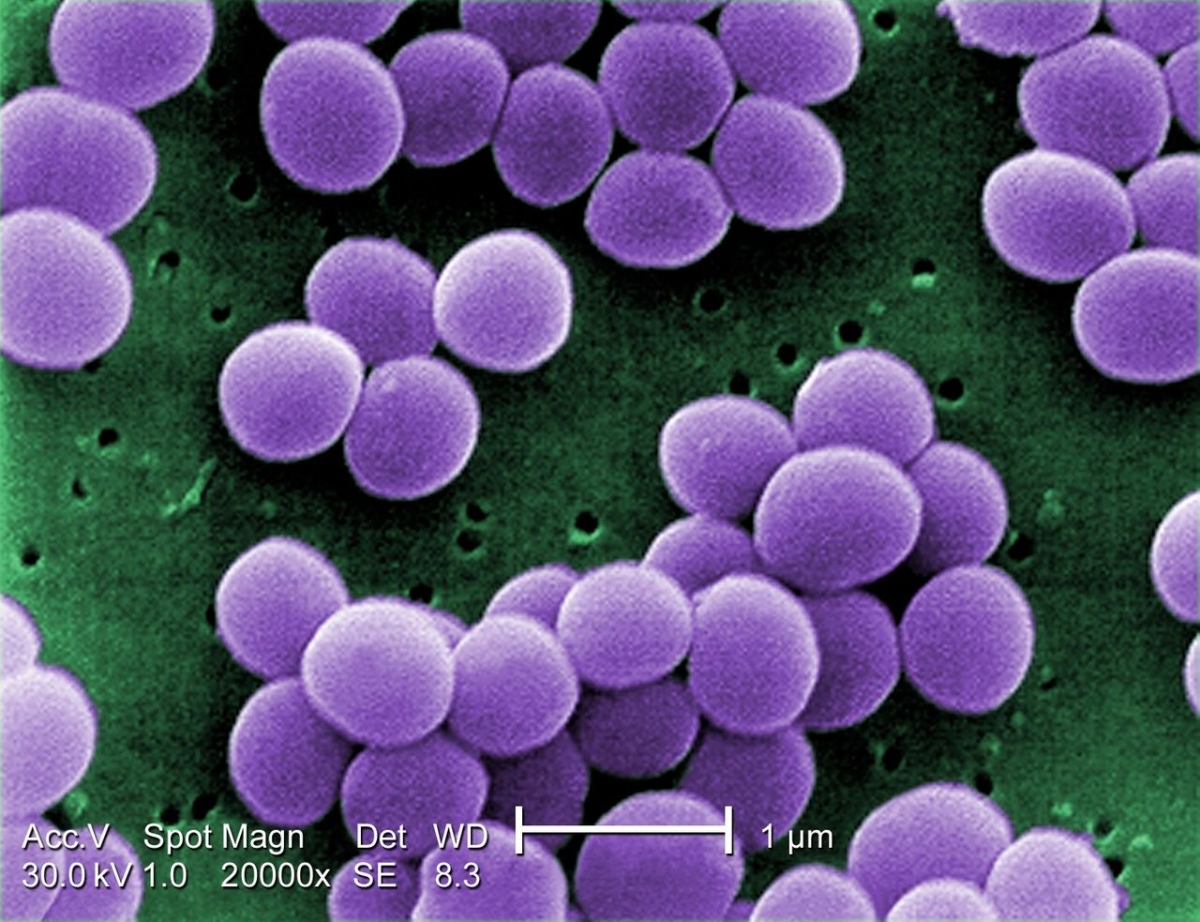 On the 18th of November, the European Antibiotic Awareness Day (EAAD) will take place, a campaign initiated by the European Centre for Disease Prevention and Control (ECDC), which is organised in partnership with the World Health Organisation (WHO) AMR Awareness week, from 18 to 24th of November. This year’s focus of the EAAD will be on the Council's Recommendation on stepping up EU actions to combat antimicrobial resistance in a One Health approach. Published in June 2023, this recommendation consists of EU strategies to combat antimicrobial resistance up to 2030 and stresses the importance of combined efforts in addressing AMR.
On the 18th of November, the European Antibiotic Awareness Day (EAAD) will take place, a campaign initiated by the European Centre for Disease Prevention and Control (ECDC), which is organised in partnership with the World Health Organisation (WHO) AMR Awareness week, from 18 to 24th of November. This year’s focus of the EAAD will be on the Council's Recommendation on stepping up EU actions to combat antimicrobial resistance in a One Health approach. Published in June 2023, this recommendation consists of EU strategies to combat antimicrobial resistance up to 2030 and stresses the importance of combined efforts in addressing AMR.
Learn more about it HERE.
EJHP: Reduce the time for medication reconciliation (MR) in the emergency department without compromising the quality.

An efficient transfer of medication history is crucial for patient safety. A recent article published in the European Journal of Hospital Pharmacy (EJHP) aimed to reduce the time used for medication reconciliation (MR) in the emergency department without compromising the quality of the MR. By restructuring the patient interview (using prescription data-based probing questions and a condensed checklist) and making documentation more concise, the time spent on MR was reduced by 34%. At the same time, the effectiveness in identifying medication discrepancies remained uncompromised. The proportion of patients with at least one identified discrepancy was similar between the revised and original methods (81% vs 79%), as was the average number of discrepancies per patient (1.9). This shows that it is possible to speed up the MR process without compromising its effectiveness in identifying medication discrepancies.
Read the article HERE.
[Consultations]
European Commission: risks of Melanoma and prevention measures related to sunbeds
In the area of prevention, the Cancer Plan announced that the Commission would explore measures on exposure to ultraviolet radiation, including from sunbeds, which increases the risk of melanoma, the most serious form of skin cancer. The call for evidence on the risks of UV-light exposure through sunbeds and how to prevent it is open to the 19th of November.
Contribute HERE.
EMA Consultation: reflection paper on the use of artificial intelligence in the lifecycle of medicines
EMA launched a draft reflection paper outlining the current thinking on the use of artificial intelligence (AI) to support the safe and effective development, regulation and use of human and veterinary medicines. The document reflects on principles relevant to the application of AI and machine learning (ML) at any step of a medicine's lifecycle, from drug discovery to the post-authorisation setting. All interested parties are invited to comment on the consultation. The public consultation is open until 31 December 2023.
Contribute HERE
EMA Consultation: guideline on clinical investigational of medicinal products in the treatment of depression
EMA launched a draft "Guideline on clinical investigation of medicinal products in the treatment of depression". The document recognises that the emergence of new antidepressants with rapid onset of effect and the repurposing of psychedelics require separate design strategies. It goes on to address key issues for the development of psychedelic-assisted psychotherapy in the field of major depressive disorder. The public consultation is open until 31 March 2024.
Contribute HERE
EFCCA Patient Preference Survey
The European Federation of Chron's & Ulcerative Colitis Associations (EFCCA) together with the University of Leuven launched the "Patient Preference Survey". It aims to find out which aspects, factors, and characteristics are important for patients when choosing a treatment for inflammatory bowel disease. The survey has been translated into several languages.
Contribute HERE




