On 14 October 2013, EAHP, together with UZ Leuven and GS1, brought together stakeholders from across the health sector spectrum: doctors' groups, nurses' groups, patient groups, representatives of the pharmacy profession, representatives of the pharmaceutical industry, and representatives of the technology, IT and health management sectors, at a one-off event to explore how to make bedside scanning in Europe a reality. Attendee list here.
Held at UZ Leuven, the event included presentations from:
- Thomas De Rijdt from UZ Leuven's Hospital Pharmacy Department on the application of bedside scanning at the hospital and its benefits for patient safety and the avoidance of medicine administration error (presentation here, video here);
- Chris Dierickx from Pfizer on the manufacturing considerations when bar coding medicines to the single unit (presentation here); and,
- Ulrike Kreysa from GS1 Healthcare on the issue of barcode standards, and the opportunities provided by the new GS1 Level Below the Each (LBE) standard (presentation here, video here).
The day also included a tour of the hospital wards to see bedside scanning technology applied in practice, and afternoon workshops at which stakeholders discussed in depth how the barriers to implementing bedside scanning more widely across Europe could be overcome. Workshop questions here.
The day ended with the presentation of findings and conclusions by the workshops, and reflection on next steps. Key conclusions were:
- Efforts towards achieving systematic bar coding of medicines to the single unit package in Europe must be better coordinated. This should bring in not only hospital pharmacy and the pharmaceutical industry, but also representatives of the packaging, software, IT and equipment industries, hospital and health system management, health insurers, and the nursing and medical professions.
- This coalition for medication safety should set out one detailed requirement in respect of the requirements of single unit package bar coding, taking into account static data versus variable data.
- This coalition should also look at the possibility of a step‐by‐step timetable for realising single unit package bar coding and give thought to constructing the timetable according to categories of medicine, whereby orphan drugs could be viewed as step 1, followed by high risk medication.
- Remaining opportunities to utilise the national implementation of the Falsified Medicines Directive should be explored.
Below you can find the formal report of the meeting, films of the presentations and links to many other relevant fields of information, including the participant list, agenda, reading materials and photo gallery.
FULL REPORT OF LEUVEN MEETING ON BEDSIDE SCANNING
Film footage of presentations:

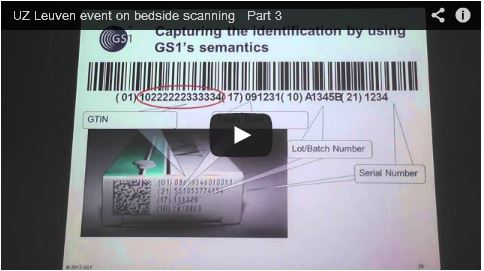

Footage of Thomas De Rijdt's presentation here.
Footage of Ulrike Kreysa's presentation here.
Footage of workshop feedback here.
Photo gallery of event:

Presentations:

Thomas De Rijdt (UZ Leuven) presentation here.
Chris Dierickx (Pfizer) presentation here.
Ulrike Kreysa (GS1) presentation here.
Background reading materials:
Further background information, studies and relevant past news related to bedside scanning and the bar coding of medicines is available here.
Contact:
For more information on how to get involved in EAHP's campaign for improved patient safety via the practice of bedside scanning, and the accompanying need to bar code medicines to the single unit package, please contact info@eahp.eu