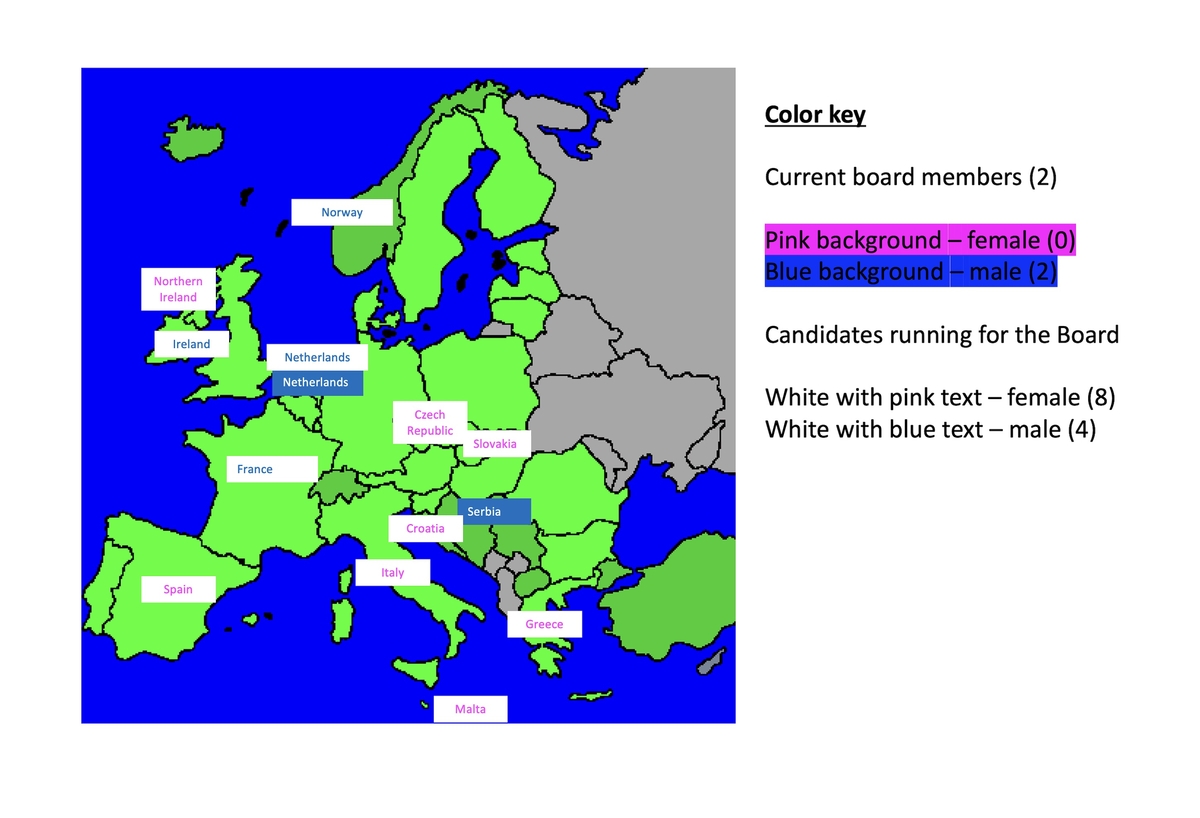
About the SIG on Automated Medication Management
 The SIG on Automated Medication Management (financially supported by Omnicell) is tasked with investigating the potential benefits of automation in the hospital setting in terms of patient safety, length of stay and staff efficiency for patient care and medication inventory management. Particular focus is put on addressing how automation in dispensing can help achieve one of the key objectives of the World Health Organisation, reducing medication errors.
The SIG on Automated Medication Management (financially supported by Omnicell) is tasked with investigating the potential benefits of automation in the hospital setting in terms of patient safety, length of stay and staff efficiency for patient care and medication inventory management. Particular focus is put on addressing how automation in dispensing can help achieve one of the key objectives of the World Health Organisation, reducing medication errors.
Europe has no uniform standard of care approach for managing all aspects of medication within the hospital setting. While some countries are further ahead than others, there are no consistent guidelines or ways to share best practices. The work of the SIG, therefore, focused on two distinct areas: mapping the current situation in Europe by conducting a survey among European Hospital Pharmacists on the use of automated medication management and creating the European autonomous pharmacy framework.
Survey on Automated Medication Management
To support the work of the SIG a survey was designed aiming at the collection of views and opinions on the current and future use of automated medication management solutions. Individual hospital pharmacists were invited to complete this survey. The survey closed on the 31st of July 2022.
The SIG Survey analysed and assessed the status of automated medication management in Europe and investigated the benefits of automating medication management, particularly around the medication preparation/compounding/dispensing process and how technology best meets the needs of different hospital pharmacy workflows.
The EAHP SIG Survey report on Automated Medication Management can be found here.
European Autonomous Pharmacy Framework (Beta Version).
The European Autonomous Pharmacy Framework was created to share a practical vision for hospital pharmacies when it comes to improving automation. The Framework consists of 4 key components: Automation, Governance of Data and Interoperability, Project Management and Workforce Allocation. Within each of the four framework components, a scale of 5 levels illustrates the progress towards full automation on certain subcategories.
The different components can be found here:
Automation
Governance of Data and Interoperability
Project Management
Workforce Allocation
European Autonomous Pharmacy Framework Tool (Beta Version).
To asses your hospitals status and progress on the European Autonomous Pharmacy Framework, a tool has been created.
Find here the tool for MAC.
Find here the tool for Windows.